Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates
- PMID: 26824680
- PMCID: PMC4854331
- DOI: 10.1088/1741-2560/13/2/026003
Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates
Abstract
Objective: Signal attenuation is a major problem facing intracortical sensors for chronic neuroprosthetic applications. Many studies suggest that failure is due to gliosis around the electrode tips, however, mechanical and material causes of failure are often overlooked. The purpose of this study was to investigate the factors contributing to progressive signal decline by using scanning electron microscopy (SEM) to visualize structural changes in chronically implanted arrays and histology to examine the tissue response at corresponding implant sites.
Approach: We examined eight chronically implanted intracortical microelectrode arrays (MEAs) explanted from non-human primates at times ranging from 37 to 1051 days post-implant. We used SEM, in vivo neural recordings, and histology (GFAP, Iba-1, NeuN). Three MEAs that were never implanted were also imaged as controls.
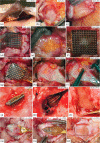
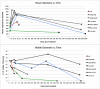
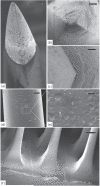
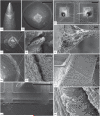
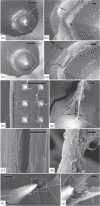
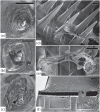
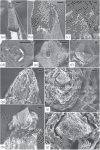
Main results: SEM revealed progressive corrosion of the platinum electrode tips and changes to the underlying silicon. The parylene insulation was prone to cracking and delamination, and in some instances the silicone elastomer also delaminated from the edges of the MEA. Substantial tissue encapsulation was observed and was often seen growing into defects in the platinum and parylene. These material defects became more common as the time in vivo increased. Histology at 37 and 1051 days post-implant showed gliosis, disruption of normal cortical architecture with minimal neuronal loss, and high Iba-1 reactivity, especially within the arachnoid and dura. Electrode tracts were either absent or barely visible in the cortex at 1051 days, but were seen in the fibrotic encapsulation material suggesting that the MEAs were lifted out of the brain. Neural recordings showed a progressive drop in impedance, signal amplitude, and viable channels over time.
Significance: These results provide evidence that signal loss in MEAs is truly multifactorial. Gliosis occurs in the first few months after implantation but does not prevent useful recordings for several years. Progressive meningeal fibrosis encapsulates and lifts MEAs out of the cortex while ongoing foreign body reactions lead to progressive degradation of the materials. Long-term impedance drops are due to the corrosion of platinum, cracking and delamination of parylene, and delamination of silicone elastomer. Oxygen radicals released by cells of the immune system likely mediate the degradation of these materials. Future MEA designs must address these problems through more durable insulation materials, more inert electrode alloys, and pharmacologic suppression of fibroblasts and leukocytes.
Figures






Similar articles
-
Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates.J Neural Eng. 2013 Dec;10(6):066014. doi: 10.1088/1741-2560/10/6/066014. Epub 2013 Nov 12. J Neural Eng. 2013. PMID: 24216311 Free PMC article.
-
Data-driven model comparing the effects of glial scarring and interface interactions on chronic neural recordings in non-human primates.J Neural Eng. 2016 Feb;13(1):016010. doi: 10.1088/1741-2560/13/1/016010. Epub 2015 Dec 14. J Neural Eng. 2016. PMID: 26655972
-
Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex.IEEE Trans Neural Syst Rehabil Eng. 2005 Dec;13(4):524-41. doi: 10.1109/TNSRE.2005.857687. IEEE Trans Neural Syst Rehabil Eng. 2005. PMID: 16425835
-
Nanostructuration strategies to enhance microelectrode array (MEA) performance for neuronal recording and stimulation.J Physiol Paris. 2012 May-Aug;106(3-4):137-45. doi: 10.1016/j.jphysparis.2011.10.001. Epub 2011 Oct 18. J Physiol Paris. 2012. PMID: 22027264 Review.
-
A review on mechanical considerations for chronically-implanted neural probes.J Neural Eng. 2018 Jun;15(3):031001. doi: 10.1088/1741-2552/aa8b4f. Epub 2017 Sep 8. J Neural Eng. 2018. PMID: 28885187 Review.
Cited by
-
Stretchable, Fully Polymeric Electrode Arrays for Peripheral Nerve Stimulation.Adv Sci (Weinh). 2021 Feb 5;8(8):2004033. doi: 10.1002/advs.202004033. eCollection 2021 Apr. Adv Sci (Weinh). 2021. PMID: 33898185 Free PMC article.
-
Chronically Implanted Intracranial Electrodes: Tissue Reaction and Electrical Changes.Micromachines (Basel). 2018 Aug 25;9(9):430. doi: 10.3390/mi9090430. Micromachines (Basel). 2018. PMID: 30424363 Free PMC article. Review.
-
Fabrication of Planar Microelectrode Array Using Laser-Patterned ITO and SU-8.Micromachines (Basel). 2021 Oct 31;12(11):1347. doi: 10.3390/mi12111347. Micromachines (Basel). 2021. PMID: 34832760 Free PMC article.
-
Toward Standardization of Electrophysiology and Computational Tissue Strain in Rodent Intracortical Microelectrode Models.Front Bioeng Biotechnol. 2020 May 8;8:416. doi: 10.3389/fbioe.2020.00416. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32457888 Free PMC article.
-
Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: new emphasis on the biological interface.J Neural Eng. 2018 Jun;15(3):033001. doi: 10.1088/1741-2552/aa9dae. Epub 2017 Nov 28. J Neural Eng. 2018. PMID: 29182149 Free PMC article.
References
-
- Hochberg LR, Donoghue JP. Sensors for brain–computer interfaces. IEEE Eng. Med. Biol. Mag. 2006;25:32–8. - PubMed
-
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. - PubMed
-
- Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nat. Neurosci. 2002;5:1085–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
