Spontaneous Ca(2+) transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine
- PMID: 26824875
- PMCID: PMC4908028
- DOI: 10.1113/JP271699
Spontaneous Ca(2+) transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine
Abstract
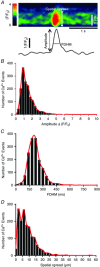
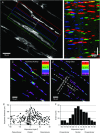
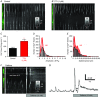
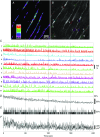
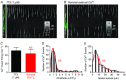
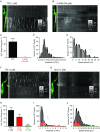
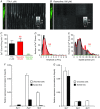
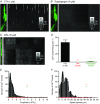
Key points: Interstitial cells of Cajal at the level of the deep muscular plexus (ICC-DMP) in the small intestine generate spontaneous Ca(2+) transients that consist of localized Ca(2+) events and limited propagating Ca(2+) waves. Ca(2+) transients in ICC-DMP display variable characteristics: from discrete, highly localized Ca(2+) transients to regionalized Ca(2+) waves with variable rates of occurrence, amplitude, duration and spatial spread. Ca(2+) transients fired stochastically, with no cellular or multicellular rhythmic activity being observed. No correlation was found between the firing sites in adjacent cells. Ca(2+) transients in ICC-DMP are suppressed by the ongoing release of inhibitory neurotransmitter(s). Functional intracellular Ca(2+) stores are essential for spontaneous Ca(2+) transients, and the sarco/endoplasmic reticulum Ca(2+) -ATPase (SERCA) pump is necessary for maintenance of spontaneity. Ca(2+) release mechanisms involve both ryanodine receptors (RyRs) and inositol triphosphate receptors (InsP3 Rs). Release from these channels is interdependent. ICC express transcripts of multiple RyRs and InsP3 Rs, with Itpr1 and Ryr2 subtypes displaying the highest expression.
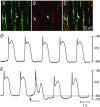
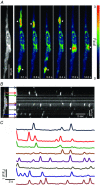
Abstract: Interstitial cells of Cajal in the deep muscular plexus of the small intestine (ICC-DMP) are closely associated with varicosities of enteric motor neurons and generate responses contributing to neural regulation of intestinal motility. Responses of ICC-DMP are mediated by activation of Ca(2+) -activated Cl(-) channels; thus, Ca(2+) signalling is central to the behaviours of these cells. Confocal imaging was used to characterize the nature and mechanisms of Ca(2+) transients in ICC-DMP within intact jejunal muscles expressing a genetically encoded Ca(2+) indicator (GCaMP3) selectively in ICC. ICC-DMP displayed spontaneous Ca(2+) transients that ranged from discrete, localized events to waves that propagated over variable distances. The occurrence of Ca(2+) transients was highly variable, and it was determined that firing was stochastic in nature. Ca(2+) transients were tabulated in multiple cells within fields of view, and no correlation was found between the events in adjacent cells. TTX (1 μm) significantly increased the occurrence of Ca(2+) transients, suggesting that ICC-DMP contributes to the tonic inhibition conveyed by ongoing activity of inhibitory motor neurons. Ca(2+) transients were minimally affected after 12 min in Ca(2+) free solution, indicating these events do not depend immediately upon Ca(2+) influx. However, inhibitors of sarco/endoplasmic reticulum Ca(2+) -ATPase (SERCA) pump and blockers of inositol triphosphate receptor (InsP3 R) and ryanodine receptor (RyR) channels blocked ICC Ca(2+) transients. These data suggest an interdependence between RyR and InsP3 R in the generation of Ca(2+) transients. Itpr1 and Ryr2 were the dominant transcripts expressed by ICC. These findings provide the first high-resolution recording of the subcellular Ca(2+) dynamics that control the behaviour of ICC-DMP in situ.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
References
-
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SR, Imaizumi Y & Nakayama S (2004). Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci 117, 2813–2825. - PubMed
-
- Beckett EA, Takeda Y, Yanase H, Sanders KM & Ward SM (2005). Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol 493, 193–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

