Receptor for Advanced Glycation End Products (RAGE) Serves a Protective Role during Klebsiella pneumoniae - Induced Pneumonia
- PMID: 26824892
- PMCID: PMC4732606
- DOI: 10.1371/journal.pone.0141000
Receptor for Advanced Glycation End Products (RAGE) Serves a Protective Role during Klebsiella pneumoniae - Induced Pneumonia
Abstract
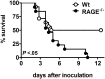
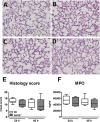
Klebsiella species is the second most commonly isolated gram-negative organism in sepsis and a frequent causative pathogen in pneumonia. The receptor for advanced glycation end products (RAGE) is expressed on different cell types and plays a key role in diverse inflammatory responses. We here aimed to investigate the role of RAGE in the host response to Klebsiella (K.) pneumoniae pneumonia and intransally inoculated rage gene deficient (RAGE-/-) and normal wild-type (Wt) mice with K. pneumoniae. Klebsiella pneumonia resulted in an increased pulmonary expression of RAGE. Furthermore, the high-affinity RAGE ligand high mobility group box-1 was upregulated during K. pneumoniae pneumonia. RAGE deficiency impaired host defense as reflected by a worsened survival, increased bacterial outgrowth and dissemination in RAGE-/- mice. RAGE-/- neutrophils showed a diminished phagocytosing capacity of live K. pneumoniae in vitro. Relative to Wt mice, RAGE-/- mice demonstrated similar lung inflammation, and slightly elevated-if any-cytokine and chemokine levels and unchanged hepatocellular injury. In addition, RAGE-/- mice displayed an unaltered response to intranasally instilled Klebsiella lipopolysaccharide (LPS) with respect to pulmonary cell recruitment and local release of cytokines and chemokines. These data suggest that (endogenous) RAGE protects against K. pneumoniae pneumonia. Also, they demonstrate that RAGE contributes to an effective antibacterial defense during K. pneumoniae pneumonia, at least partly via its participation in the phagocytic properties of professional granulocytes. Additionally, our results indicate that RAGE is not essential for the induction of a local and systemic inflammatory response to either intact Klebsiella or Klebsiella LPS.
Conflict of interest statement
Figures



References
-
- Kollef MH, Kollef KE (2005) Antibiotic utilization and outcomes for patients with clinically suspected ventilator-associated pneumonia and negative quantitative BAL culture results. Chest 128: 2706–2713. - PubMed
-
- Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH (2006) Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database. Crit Care Med 34: 2588–2595. - PubMed
-
- Giamarellou H (2005) Multidrug resistance in Gram-negative bacteria that produce extended-spectrum beta-lactamases (ESBLs). Clin Microbiol Infect 11 Suppl 4: 1–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

