Intrahepatic Tissue Implantation Represents a Favorable Approach for Establishing Orthotopic Transplantation Hepatocellular Carcinoma Mouse Models
- PMID: 26824903
- PMCID: PMC4732811
- DOI: 10.1371/journal.pone.0148263
Intrahepatic Tissue Implantation Represents a Favorable Approach for Establishing Orthotopic Transplantation Hepatocellular Carcinoma Mouse Models
Erratum in
-
Correction: Intrahepatic Tissue Implantation Represents a Favorable Approach for Establishing Orthotopic Transplantation Hepatocellular Carcinoma Mouse Models.PLoS One. 2018 Oct 18;13(10):e0206322. doi: 10.1371/journal.pone.0206322. eCollection 2018. PLoS One. 2018. PMID: 30335849 Free PMC article.
Abstract
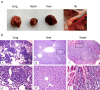
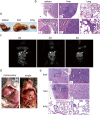
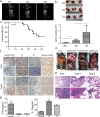
Mouse models are commonly used for studying hepatocellular carcinoma (HCC) biology and exploring new therapeutic interventions. Currently three main modalities of HCC mouse models have been extensively employed in pre-clinical studies including chemically induced, transgenic and transplantation models. Among them, transplantation models are preferred for evaluating in vivo drug efficacy in pre-clinical settings given the short latency, uniformity in size and close resemblance to tumors in patients. However methods used for establishing orthotopic HCC transplantation mouse models are diverse and fragmentized without a comprehensive comparison. Here, we systemically evaluate four different approaches commonly used to establish HCC mice in preclinical studies, including intravenous, intrasplenic, intrahepatic inoculation of tumor cells and intrahepatic tissue implantation. Four parameters--the latency period, take rates, pathological features and metastatic rates--were evaluated side-by-side. 100% take rates were achieved in liver with intrahepatic, intrasplenic inoculation of tumor cells and intrahepatic tissue implantation. In contrast, no tumor in liver was observed with intravenous injection of tumor cells. Intrahepatic tissue implantation resulted in the shortest latency with 0.5 cm (longitudinal diameter) tumors found in liver two weeks after implantation, compared to 0.1cm for intrahepatic inoculation of tumor cells. Approximately 0.1cm tumors were only visible at 4 weeks after intrasplenic inoculation. Uniform, focal and solitary tumors were formed with intrahepatic tissue implantation whereas multinodular, dispersed and non-uniform tumors produced with intrahepatic and intrasplenic inoculation of tumor cells. Notably, metastasis became visible in liver, peritoneum and mesenterium at 3 weeks post-implantation, and lung metastasis was visible after 7 weeks. T cell infiltration was evident in tumors, resembling the situation in HCC patients. Our study demonstrated that orthotopic HCC mouse models established via intrahepatic tissue implantation authentically reflect clinical manifestations in HCC patients pathologically and immunologically, suggesting intrahepatic tissue implantation is a preferable approach for establishing orthotopic HCC mouse models.
Conflict of interest statement
Figures
Similar articles
-
[Establishment of human hepatocellular carcinoma cell line with spontaneous pulmonary metastasis through in vivo selection].Zhonghua Yi Xue Za Zhi. 2002 May 10;82(9):601-5. Zhonghua Yi Xue Za Zhi. 2002. PMID: 12133480 Chinese.
-
Nude mice model of human hepatocellular carcinoma via orthotopic implantation of histologically intact tissue.World J Gastroenterol. 2004 Nov 1;10(21):3107-11. doi: 10.3748/wjg.v10.i21.3107. World J Gastroenterol. 2004. PMID: 15457553 Free PMC article.
-
[Highly metastatic model of human hepatocellular carcinoma established in nude mice using orthotopic organ selection of metastatic variant from patient specimens].Zhonghua Zhong Liu Za Zhi. 1996 Mar;18(2):109-12. Zhonghua Zhong Liu Za Zhi. 1996. PMID: 9206041 Chinese.
-
Metastatic human hepatocellular carcinoma models in nude mice and cell line with metastatic potential.World J Gastroenterol. 2001 Oct;7(5):597-601. doi: 10.3748/wjg.v7.i5.597. World J Gastroenterol. 2001. PMID: 11819839 Free PMC article. Review.
-
Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis.Cancer Metastasis Rev. 1991 Oct;10(3):229-43. doi: 10.1007/BF00050794. Cancer Metastasis Rev. 1991. PMID: 1764766 Review.
Cited by
-
Correction: Intrahepatic Tissue Implantation Represents a Favorable Approach for Establishing Orthotopic Transplantation Hepatocellular Carcinoma Mouse Models.PLoS One. 2018 Oct 18;13(10):e0206322. doi: 10.1371/journal.pone.0206322. eCollection 2018. PLoS One. 2018. PMID: 30335849 Free PMC article.
-
Patient-derived models facilitate precision medicine in liver cancer by remodeling cell-matrix interaction.Front Immunol. 2023 May 4;14:1101324. doi: 10.3389/fimmu.2023.1101324. eCollection 2023. Front Immunol. 2023. PMID: 37215109 Free PMC article. Review.
-
Validation of Hepatocellular Carcinoma Experimental Models for TGF-β Promoting Tumor Progression.Cancers (Basel). 2019 Oct 9;11(10):1510. doi: 10.3390/cancers11101510. Cancers (Basel). 2019. PMID: 31600917 Free PMC article. Review.
-
Spontaneous and Induced Animal Models for Cancer Research.Diagnostics (Basel). 2020 Aug 31;10(9):660. doi: 10.3390/diagnostics10090660. Diagnostics (Basel). 2020. PMID: 32878340 Free PMC article. Review.
-
ESTABLISHMENT AND EVALUATION OF ORTHOTOPIC HEPATOCELLULAR CARCINOMA AND DRUG-INDUCED HEPATOCELLULAR CARCINOMA IN MICE WITH SPLEEN-DEFICIENCY SYNDROME IN TRADITIONAL CHINESE MEDICINE.Afr J Tradit Complement Altern Med. 2016 Nov 23;14(1):165-173. doi: 10.21010/ajtcam.v14i1.18. eCollection 2017. Afr J Tradit Complement Altern Med. 2016. PMID: 28480394 Free PMC article.
References
-
- Foster JR. Spontaneous and drug-induced hepatic pathology of the laboratory beagle dog, the cynomolgus macaque and the marmoset. Toxicol Pathol. 2005; 33: 63–74. - PubMed
-
- Li Y, Tang ZY, Hou JX. Hepatocellular carcinoma: insight from animal models. Nat Rev Gastroenterol Hepatol. 2012; 9: 32–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

