AMP-Activated Protein Kinase Regulates Oxidative Metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 Transcriptional Regulators
- PMID: 26824904
- PMCID: PMC4732773
- DOI: 10.1371/journal.pone.0148089
AMP-Activated Protein Kinase Regulates Oxidative Metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 Transcriptional Regulators
Abstract
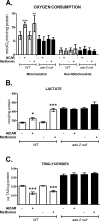
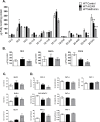
Cellular energy regulation relies on complex signaling pathways that respond to fuel availability and metabolic demands. Dysregulation of these networks is implicated in the development of human metabolic diseases such as obesity and metabolic syndrome. In Caenorhabditis elegans the AMP-activated protein kinase, AAK, has been associated with longevity and stress resistance; nevertheless its precise role in energy metabolism remains elusive. In the present study, we find an evolutionary conserved role of AAK in oxidative metabolism. Similar to mammals, AAK is activated by AICAR and metformin and leads to increased glycolytic and oxidative metabolic fluxes evidenced by an increase in lactate levels and mitochondrial oxygen consumption and a decrease in total fatty acids and lipid storage, whereas augmented glucose availability has the opposite effects. We found that these changes were largely dependent on the catalytic subunit AAK-2, since the aak-2 null strain lost the observed metabolic actions. Further results demonstrate that the effects due to AAK activation are associated to SBP-1 and NHR-49 transcriptional factors and MDT-15 transcriptional co-activator, suggesting a regulatory pathway that controls oxidative metabolism. Our findings establish C. elegans as a tractable model system to dissect the relationship between distinct molecules that play a critical role in the regulation of energy metabolism in human metabolic diseases.
Conflict of interest statement
Figures





Similar articles
-
The conserved Mediator subunit MDT-15 is required for oxidative stress responses in Caenorhabditis elegans.Aging Cell. 2014 Feb;13(1):70-9. doi: 10.1111/acel.12154. Epub 2013 Sep 18. Aging Cell. 2014. PMID: 23957350 Free PMC article.
-
Transcription factors CEP-1/p53 and CEH-23 collaborate with AAK-2/AMPK to modulate longevity in Caenorhabditis elegans.Aging Cell. 2017 Aug;16(4):814-824. doi: 10.1111/acel.12619. Epub 2017 May 30. Aging Cell. 2017. PMID: 28560849 Free PMC article.
-
5'-AMP-Activated Protein Kinase Signaling in Caenorhabditis elegans.Exp Suppl. 2016;107:375-388. doi: 10.1007/978-3-319-43589-3_15. Exp Suppl. 2016. PMID: 27812988 Review.
-
A novel peptide derived from Haematococcus pluvialis residue balanced lipid metabolism through NHR-49/PPARα and AAK-2/AMPK pathways in Caenorhabditis elegans.Biofactors. 2025 Mar-Apr;51(2):e70017. doi: 10.1002/biof.70017. Biofactors. 2025. PMID: 40249062
-
5'-AMP-activated protein kinase signaling in Caenorhabditis elegans.Exp Biol Med (Maywood). 2008 Jan;233(1):12-20. doi: 10.3181/0705-MR-117. Exp Biol Med (Maywood). 2008. PMID: 18156301 Review.
Cited by
-
Maackiain Mimics Caloric Restriction through aak-2-Mediated Lipid Reduction in Caenorhabditis elegans.Int J Mol Sci. 2023 Dec 13;24(24):17442. doi: 10.3390/ijms242417442. Int J Mol Sci. 2023. PMID: 38139270 Free PMC article.
-
Sedentary Lifestyles and a Hypercaloric Diets During Middle Age, are Binomial Conducive to Fatal Progression, That is Counteracted by the Hormetic Treatment of Exercise, Metformin, and Tert-Butyl Hydroquinone: An Analysis of Female Middle-Aged Rat Liver Mitochondria.Dose Response. 2024 Oct 10;22(4):15593258241272619. doi: 10.1177/15593258241272619. eCollection 2024 Oct-Dec. Dose Response. 2024. PMID: 39399210 Free PMC article.
-
A high glucose diet induces autophagy in a HLH-30/TFEB-dependent manner and impairs the normal lifespan of C. elegans.Aging (Albany NY). 2018 Oct 5;10(10):2657-2667. doi: 10.18632/aging.101577. Aging (Albany NY). 2018. PMID: 30299269 Free PMC article.
-
Purine Homeostasis Is Necessary for Developmental Timing, Germline Maintenance and Muscle Integrity in Caenorhabditis elegans.Genetics. 2019 Apr;211(4):1297-1313. doi: 10.1534/genetics.118.301062. Epub 2019 Jan 30. Genetics. 2019. PMID: 30700528 Free PMC article.
-
Effects of High Dietary Carbohydrate and Lipid Intake on the Lifespan of C. elegans.Cells. 2021 Sep 8;10(9):2359. doi: 10.3390/cells10092359. Cells. 2021. PMID: 34572007 Free PMC article. Review.
References
-
- Hamilton MT, Hamilton DG, Zderic TW (2007) Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667. - PubMed
-
- Elle IC, Olsen LCB, Mosbech M-B, Rødkær SV, Pultz D, Boelt SG, et al. (2008) C. elegans: A Model for Understanding Lipid Accumulation. Lipid Insights 1: 13–21.
-
- Moreno-Arriola E, Cardenas-Rodriguez N, Coballase-Urrutia E, Pedraza-Chaverri J, Carmona-Aparicio L, Ortega-Cuellar D (2014) Caenorhabditis elegans: A useful model for studying metabolic disorders in which oxidative stress is a contributing factor. Oxid Med Cell Longev 2014: 705253 10.1155/2014/705253 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

