Mechanisms of in vivo muscle fatigue in humans: investigating age-related fatigue resistance with a computational model
- PMID: 26824934
- PMCID: PMC4908014
- DOI: 10.1113/JP271400
Mechanisms of in vivo muscle fatigue in humans: investigating age-related fatigue resistance with a computational model
Abstract
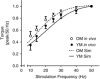
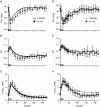
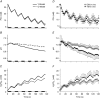
Key points: Muscle fatigue can be defined as the transient decrease in maximal force that occurs in response to muscle use. Fatigue develops because of a complex set of changes within the neuromuscular system that are difficult to evaluate simultaneously in humans. The skeletal muscle of older adults fatigues less than that of young adults during static contractions. The potential sources of this difference are multiple and intertwined. To evaluate the individual mechanisms of fatigue, we developed an integrative computational model based on neural, biochemical, morphological and physiological properties of human skeletal muscle. Our results indicate first that the model provides accurate predictions of fatigue and second that the age-related resistance to fatigue is due largely to a lower reliance on glycolytic metabolism during contraction. This model should prove useful for generating hypotheses for future experimental studies into the mechanisms of muscle fatigue.
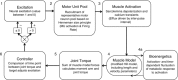
Abstract: During repeated or sustained muscle activation, force-generating capacity becomes limited in a process referred to as fatigue. Multiple factors, including motor unit activation patterns, muscle fibre contractile properties and bioenergetic function, can impact force-generating capacity and thus the potential to resist fatigue. Given that neuromuscular fatigue depends on interrelated factors, quantifying their independent effects on force-generating capacity is not possible in vivo. Computational models can provide insight into complex systems in which multiple inputs determine discrete outputs. However, few computational models to date have investigated neuromuscular fatigue by incorporating the multiple levels of neuromuscular function known to impact human in vivo function. To address this limitation, we present a computational model that predicts neural activation, biomechanical forces, intracellular metabolic perturbations and, ultimately, fatigue during repeated isometric contractions. This model was compared with metabolic and contractile responses to repeated activation using values reported in the literature. Once validated in this way, the model was modified to reflect age-related changes in neuromuscular function. Comparisons between initial and age-modified simulations indicated that the age-modified model predicted less fatigue during repeated isometric contractions, consistent with reports in the literature. Together, our simulations suggest that reduced glycolytic flux is the greatest contributor to the phenomenon of age-related fatigue resistance. In contrast, oxidative resynthesis of phosphocreatine between intermittent contractions and inherent buffering capacity had minimal impact on predicted fatigue during isometric contractions. The insights gained from these simulations cannot be achieved through traditional in vivo or in vitro experimentation alone.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
Similar articles
-
Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks.J Appl Physiol (1985). 2004 Sep;97(3):967-75. doi: 10.1152/japplphysiol.01351.2003. Epub 2004 May 14. J Appl Physiol (1985). 2004. PMID: 15145914 Clinical Trial.
-
Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow.J Physiol. 2007 Sep 15;583(Pt 3):1093-105. doi: 10.1113/jphysiol.2007.138362. Epub 2007 Aug 2. J Physiol. 2007. PMID: 17673506 Free PMC article.
-
A motor unit-based model of muscle fatigue.PLoS Comput Biol. 2017 Jun 2;13(6):e1005581. doi: 10.1371/journal.pcbi.1005581. eCollection 2017 Jun. PLoS Comput Biol. 2017. PMID: 28574981 Free PMC article.
-
Residual force enhancement in humans: Current evidence and unresolved issues.J Electromyogr Kinesiol. 2015 Aug;25(4):571-80. doi: 10.1016/j.jelekin.2015.04.011. Epub 2015 Apr 23. J Electromyogr Kinesiol. 2015. PMID: 25956547 Review.
-
Neuromuscular fatigue and aging: central and peripheral factors.Muscle Nerve. 2002 Jun;25(6):785-96. doi: 10.1002/mus.10116. Muscle Nerve. 2002. PMID: 12115966 Review.
Cited by
-
Monitoring the training dose and acute fatigue response during elbow flexor resistance training using a custom-made resistance band.PeerJ. 2020 Feb 25;8:e8689. doi: 10.7717/peerj.8689. eCollection 2020. PeerJ. 2020. PMID: 32140314 Free PMC article.
-
The impact of submaximal fatiguing exercises on the ability to generate and sustain the maximal voluntary contraction.Front Physiol. 2022 Sep 2;13:970917. doi: 10.3389/fphys.2022.970917. eCollection 2022. Front Physiol. 2022. PMID: 36117706 Free PMC article.
-
The effect of soft tissue manipulation and rest on knee extensor muscles fatigue: Do torque parameters and induced perception following muscle fatigue have enough reliability?J Family Med Prim Care. 2020 Feb 28;9(2):950-956. doi: 10.4103/jfmpc.jfmpc_838_19. eCollection 2020 Feb. J Family Med Prim Care. 2020. PMID: 32318451 Free PMC article.
-
Exercise Physiology From 1980 to 2020: Application of the Natural Sciences.Kinesiol Rev (Champaign). 2021 Aug;10(3):238-247. doi: 10.1123/kr.2021-0024. Epub 2021 Jun 30. Kinesiol Rev (Champaign). 2021. PMID: 35464337 Free PMC article.
-
From Simulation to Reality: Predicting Torque With Fatigue Onset via Transfer Learning.IEEE Trans Neural Syst Rehabil Eng. 2024;32:3669-3676. doi: 10.1109/TNSRE.2024.3465016. Epub 2024 Oct 7. IEEE Trans Neural Syst Rehabil Eng. 2024. PMID: 39302781 Free PMC article.
References
-
- Allman BL, Cheng AJ & Rice CL (2004). Quadriceps fatigue caused by catchlike‐inducing trains is not altered in old age. Muscle Nerve 30, 743–751. - PubMed
-
- Bazzucchi I, Marchetti M, Rosponi A, Fattorini L, Castellano V, Sbriccoli P & Felici F (2005). Differences in the force/endurance relationship between young and older men. Eur J Appl Physiol 93, 390–397. - PubMed
-
- Bilodeau M, Erb MD, Nichols JM, Joiner KL & Weeks JB (2001). Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve 24, 98–106. - PubMed
-
- Calderon JC, Bolanos P, Torres SH, Rodriguez‐Arroyo G & Caputo C (2009). Different fibre populations distinguished by their calcium transient characteristics in enzymatically dissociated murine flexor digitorum brevis and soleus muscles. J Muscle Res Cell Motil 30, 125–137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

