Mutational Analysis of Quinolone Resistance Protein QnrVC7 Provides Novel Insights into the Structure-Activity Relationship of Qnr Proteins
- PMID: 26824937
- PMCID: PMC4775992
- DOI: 10.1128/AAC.01805-15
Mutational Analysis of Quinolone Resistance Protein QnrVC7 Provides Novel Insights into the Structure-Activity Relationship of Qnr Proteins
Abstract
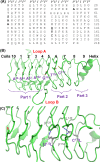
This study assessed the functional importance of residues located at the i(-2) position of face 4 of the tandem repeat loops of the quinolone resistance protein QnrVC7 through mutagenesis studies. The i(-2) position of face 4 on different coils required residues with different natures. Some substitutions reduced the protective activity of QnrVC7, while some of them increased it. These findings advanced our understanding on the detailed structural organization and functional requirements of Qnr proteins.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Lahey Clinic. 2015. qnr numbering and sequence. Lahey Clinic, Burlington, MA: http://www.lahey.org/qnrstudies/.
-
- Xiong X, Bromley EH, Oelschlaeger P, Woolfson DN, Spencer J. 2011. Structural insights into quinolone antibiotic resistance mediated by pentapeptide repeat proteins: conserved surface loops direct the activity of a Qnr protein from a Gram-negative bacterium. Nucleic Acids Res 39:3917–3927. doi: 10.1093/nar/gkq1296. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

