Contribution of Population Pharmacokinetics to Dose Optimization of Ganciclovir-Valganciclovir in Solid-Organ Transplant Patients
- PMID: 26824942
- PMCID: PMC4808182
- DOI: 10.1128/AAC.02130-15
Contribution of Population Pharmacokinetics to Dose Optimization of Ganciclovir-Valganciclovir in Solid-Organ Transplant Patients
Abstract
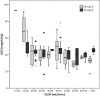
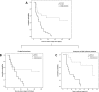
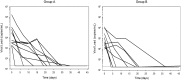
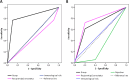
Treatment of solid-organ transplant (SOT) patients with ganciclovir (GCV)-valganciclovir (VGCV) according to the manufacturer's recommendations may result in over- or underexposure. Bayesian prediction based on a population pharmacokinetics model may optimize GCV-VGCV dosing, achieving the area under the curve (AUC) therapeutic target. We conducted a two-arm, randomized, open-label, 40% superiority trial in adult SOT patients receiving GCV-VGCV as prophylaxis or treatment of cytomegalovirus infection. Group A was treated according to the manufacturer's recommendations. For group B, the dosing was adjusted based on target exposures using a Bayesian prediction model (NONMEM). Fifty-three patients were recruited (27 in group A and 26 in group B). About 88.6% of patients in group B and 22.2% in group A reached target AUC, achieving the 40% superiority margin (P< 0.001; 95% confidence interval [CI] difference, 47 to 86%). The time to reach target AUC was significantly longer in group A than in group B (55.9 ± 8.2 versus 15.8 ± 2.3 days,P< 0.001). A shorter time to viral clearance was observed in group B than in group A (12.5 versus 17.6 days;P= 0.125). The incidences of relapse (group A, 66.67%, and group B, 9.01%) and late-onset infection (group A, 36.7%, and group B, 7.7%) were higher in group A. Neutropenia and anemia were related to GCV overexposure. GCV-VCGV dose adjustment based on a population pharmacokinetics Bayesian prediction model optimizes GCV-VGCV exposure. (This study has been registered at ClinicalTrials.gov under registration no. NCT01446445.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- de la Torre-Cisneros J, Fariñas MC, Castón JJ, Aguado JM, Cantisán S, Carratalá J, Cervera C, Cisneros JM, Cordero E, Crespo-Leiro MG, Fortún J, Frauca E, Gavaldá J, Gil-Vernet S, Gurguí M, Len O, Lumbreras C, Marcos MÁ, Martín-Dávila P, Monforte V, Montejo M, Moreno A, Muñoz P, Navarro D, Pahissa A, Pérez JL, Rodriguez-Bernot A, Rumbao J, San Juan R, Santos F, Varo E, Zurbano F, GESITRA-SEIMC/REIPI . 2011. GESITRA-SEIMC/REIPI recommendations for the management of cytomegalovirus infection in solid-organ transplant patients. Enferm Infecc Microbiol Clin 29:735–758. doi:10.1016/j.eimc.2011.05.022. - DOI - PubMed
-
- Cervera C, Fernández-Ruiz M, Valledor A, Linares L, Antón A, Ángeles Marcos M, Sanclemente G, Hoyo I, Cofán F, Ricart MJ, Pérez-Villa F, Navasa M, Pumarola T, Moreno A. 2011. Epidemiology and risk factors for late infection in solid organ transplant recipients. Transpl Infect Dis 13:598–607. doi:10.1111/j.1399-3062.2011.00646.x. - DOI - PubMed
-
- Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, Squifflet JP, Vialtel P, Abramowicz D, Mourad G, Wolf P, Cassuto E, Moulin B, Rifle G, Pruna A, Merville P, Mignon F, Legendre C, Le Pogamp P, Lebranchu Y, Toupance O, Hurault De Ligny B, Touchard G, Olmer M, Purgus R, Pouteil-Noble C, Glotz D, Bourbigot B, Leski M, Wauters JP, Kessler M. 2003. A three arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 75:844–851. doi:10.1097/01.TP.0000056635.59888.EF. - DOI - PubMed
-
- Tolkoff-Rubin NE, Rubin RH. 1999. The impact of cytomegalovirus infection on graft function and patient outcome. Graft 2:S101–S103.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

