Nitazoxanide Inhibits Pilus Biogenesis by Interfering with Folding of the Usher Protein in the Outer Membrane
- PMID: 26824945
- PMCID: PMC4808142
- DOI: 10.1128/AAC.02221-15
Nitazoxanide Inhibits Pilus Biogenesis by Interfering with Folding of the Usher Protein in the Outer Membrane
Abstract
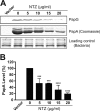
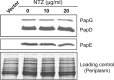
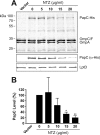
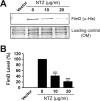
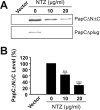
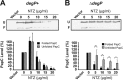
Many bacterial pathogens assemble surface fibers termed pili or fimbriae that facilitate attachment to host cells and colonization of host tissues. The chaperone/usher (CU) pathway is a conserved secretion system that is responsible for the assembly of virulence-associated pili by many different Gram-negative bacteria. Pilus biogenesis by the CU pathway requires a dedicated periplasmic chaperone and an integral outer membrane (OM) assembly and secretion platform termed the usher. Nitazoxanide (NTZ), an antiparasitic drug, was previously shown to inhibit the function of aggregative adherence fimbriae and type 1 pili assembled by the CU pathway in enteroaggregativeEscherichia coli, an important causative agent of diarrhea. We show here that NTZ also inhibits the function of type 1 and P pili from uropathogenicE. coli(UPEC). UPEC is the primary causative agent of urinary tract infections, and type 1 and P pili mediate colonization of the bladder and kidneys, respectively. By analysis of the different stages of the CU pilus biogenesis pathway, we show that treatment of bacteria with NTZ causes a reduction in the number of usher molecules in the OM, resulting in a loss of pilus assembly on the bacterial surface. In addition, we determine that NTZ specifically prevents proper folding of the usher β-barrel domain in the OM. Our findings demonstrate that NTZ is a pilicide with a novel mechanism of action and activity against diverse CU pathways. This suggests that further development of the NTZ scaffold may lead to new antivirulence agents that target the usher to prevent pilus assembly.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
The small molecule nitazoxanide selectively disrupts BAM-mediated folding of the outer membrane usher protein.J Biol Chem. 2019 Sep 27;294(39):14357-14369. doi: 10.1074/jbc.RA119.009616. Epub 2019 Aug 7. J Biol Chem. 2019. PMID: 31391254 Free PMC article.
-
Therapeutic Approaches Targeting the Assembly and Function of Chaperone-Usher Pili.EcoSal Plus. 2019 Mar;8(2):10.1128/ecosalplus.ESP-0033-2018. doi: 10.1128/ecosalplus.ESP-0033-2018. EcoSal Plus. 2019. PMID: 30873935 Free PMC article. Review.
-
The Escherichia coli P and Type 1 Pilus Assembly Chaperones PapD and FimC Are Monomeric in Solution.J Bacteriol. 2016 Aug 11;198(17):2360-9. doi: 10.1128/JB.00366-16. Print 2016 Sep 1. J Bacteriol. 2016. PMID: 27353649 Free PMC article.
-
Nitazoxanide inhibits pili assembly by targeting BamB to synergize with polymyxin B against drug-resistant Escherichia coli.Biochimie. 2025 Jun;233:47-59. doi: 10.1016/j.biochi.2025.02.006. Epub 2025 Feb 19. Biochimie. 2025. PMID: 39984113
-
Pili Assembled by the Chaperone/Usher Pathway in Escherichia coli and Salmonella.EcoSal Plus. 2018 Mar;8(1):10.1128/ecosalplus.ESP-0007-2017. doi: 10.1128/ecosalplus.ESP-0007-2017. EcoSal Plus. 2018. PMID: 29536829 Free PMC article. Review.
Cited by
-
Chaperone-tip adhesin complex is vital for synergistic activation of CFA/I fimbriae biogenesis.PLoS Pathog. 2020 Oct 2;16(10):e1008848. doi: 10.1371/journal.ppat.1008848. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33007034 Free PMC article.
-
Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects.Res Rep Urol. 2022 Apr 4;14:109-133. doi: 10.2147/RRU.S273663. eCollection 2022. Res Rep Urol. 2022. PMID: 35402319 Free PMC article. Review.
-
The Bacteriology and Its Virulence Factors in Neonatal Infections: Threats to Child Survival Strategies.J Pathog. 2018 Jul 2;2018:4801247. doi: 10.1155/2018/4801247. eCollection 2018. J Pathog. 2018. PMID: 30112215 Free PMC article.
-
Small Molecules That Sabotage Bacterial Virulence.Trends Pharmacol Sci. 2017 Apr;38(4):339-362. doi: 10.1016/j.tips.2017.01.004. Epub 2017 Feb 14. Trends Pharmacol Sci. 2017. PMID: 28209403 Free PMC article. Review.
-
Amixicile, a novel strategy for targeting oral anaerobic pathogens.Sci Rep. 2017 Sep 5;7(1):10474. doi: 10.1038/s41598-017-09616-0. Sci Rep. 2017. PMID: 28874750 Free PMC article.
References
-
- Cusumano CK, Hultgren SJ. 2009. Bacterial adhesion—a source of alternate antibiotic targets. IDrugs 12:699–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

