Innovations for phase I dose-finding designs in pediatric oncology clinical trials
- PMID: 26825023
- PMCID: PMC4818190
- DOI: 10.1016/j.cct.2016.01.009
Innovations for phase I dose-finding designs in pediatric oncology clinical trials
Abstract
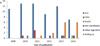
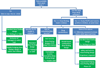
Phase I oncology clinical trials are designed to identify the optimal dose that will be recommended for phase II trials. In pediatric oncology, the conduct of those trials raises specific challenges, as the disease is rare with limited therapeutic options. In addition, the tolerance profile is known from adult trials. This paper provides a review of the major recent developments in the design of these trials, inspired by the need to cope with the specific challenges of dose finding in cancer pediatric oncology. We reviewed simulation studies comparing designs dedicated to address these challenges. We also reviewed the design used in published dose-finding trials in pediatric oncology over the period 2009-2014. Three main fields of innovation were identified. First, designs that were developed in order to relax the rules for more flexible inclusions. Second, methods to incorporate data emerging from adult studies. Third, designs accounting for toxicity evaluation at repeated cycles in pediatric oncology. In addition to this overview, we propose some further directions for designing pediatric dose-finding trials.
Keywords: Adaptive designs; Dose-finding clinical trials; Oncology; Pediatrics; Phase I; Review.
Published by Elsevier Inc.
Figures


References
-
- Lee DP, Skolnik JM, Adamson PC. Pediatric phase I trials in oncology: an analysis of study conduct efficiency. J Clin Oncol. 2005;23(33):8431–8441. - PubMed
-
- Paoletti X, Geoerger B, Doz F, Baruchel A, Lokiec F, Le Tourneau C. A comparative analysis of paediatric dose-finding trials of molecularly targeted agent with adults' trials. Eur J Cancer. 2013;49(10):2392–2402. - PubMed
-
- Smith M, Ho PT. Pediatric drug development: a perspective from the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) Invest New Drugs. 1996;14(1):11–22. - PubMed
-
- Estlin EJ, Cotterill S, Pratt CB, Pearson AD, Bernstein M. Phase I trials in pediatric oncology: perceptions of pediatricians from the United Kingdom Children's Cancer Study Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18(9):1900–1905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

