Identification of new palmitoylated proteins in Toxoplasma gondii
- PMID: 26825284
- PMCID: PMC4857766
- DOI: 10.1016/j.bbapap.2016.01.010
Identification of new palmitoylated proteins in Toxoplasma gondii
Abstract
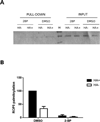
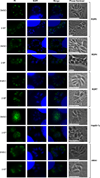
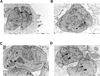
Protein palmitoylation has been shown to be an important post-translational modification in eukaryotic cells. This modification alters the localization and/or the function of the targeted protein. In recent years, protein palmitoylation has risen in importance in apicomplexan parasites as well. In Toxoplasma gondii, some proteins have been reported to be modified by palmitate. With the development of new techniques that allow the isolation of palmitoylated proteins, this significant post-translational modification has begun to be studied in more detail in T. gondii. Here we describe the palmitoylome of the tachyzoite stage of T. gondii using a combination of the acyl-biotin exchange chemistry method and mass spectrometry analysis. We identified 401 proteins found in multiple cellular compartments, with a wide range of functions that vary from metabolic processes, gliding and host-cell invasion to even regulation of transcription and translation. Besides, we found that more rhoptry proteins than the ones already described for Toxoplasma are palmitoylated, suggesting an important role for this modification in the invasion mechanism of the host-cell. This study documents that protein palmitoylation is a common modification in T. gondii that could have an impact on different cellular processes.
Keywords: Acyl-biotin exchange; Host-cell invasion; Palmitoylome; Protein identification; Rhoptry; Toxoplasma gondii.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures


References
-
- Linder ME, Deschenes RJ. New insights into the mechanisms of protein palmitoylation. Biochemistry. 2003;42:4311–4320. - PubMed
-
- Berthiaume L, Deichaite I, Peseckis S, Resh MD. Regulation of enzymatic activity by active site fatty acylation. A new role for long chain fatty acid acylation of proteins. J Biol Chem. 1994;269:6498–6505. - PubMed
-
- Corvi MM, Soltys CL, Berthiaume LG. Regulation of mitochondrial carbamoyl-phosphate synthetase 1 activity by active site fatty acylation. J Biol Chem. 2001;276:45704–45712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

