Thymic stromal cell subsets for T cell development
- PMID: 26825337
- PMCID: PMC11108406
- DOI: 10.1007/s00018-015-2107-8
Thymic stromal cell subsets for T cell development
Abstract
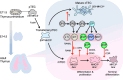
The thymus provides a specialized microenvironment in which a variety of stromal cells of both hematopoietic and non-hematopoietic origin regulate development and repertoire selection of T cells. Recent studies have been unraveling the inter- and intracellular signals and transcriptional networks for spatiotemporal regulation of development of thymic stromal cells, mainly thymic epithelial cells (TECs), and the molecular mechanisms of how different TEC subsets control T cell development and selection. TECs are classified into two functionally different subsets: cortical TECs (cTECs) and medullary TECs (mTECs). cTECs induce positive selection of diverse and functionally distinct T cells by virtue of unique antigen-processing systems, while mTECs are essential for establishing T cell tolerance via ectopic expression of peripheral tissue-restricted antigens and cooperation with dendritic cells. In addition to reviewing the role of the thymic stroma in conventional T cell development, we will discuss recently discovered novel functions of TECs in the development of unconventional T cells, such as natural killer T cells and γδT cells.
Keywords: Repertoire selection; T cell; Thymic epithelial cell; Thymus; cTEC; mTEC.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

