Lipoprotein(a): novel target and emergence of novel therapies to lower cardiovascular disease risk
- PMID: 26825471
- PMCID: PMC5061509
- DOI: 10.1097/MED.0000000000000237
Lipoprotein(a): novel target and emergence of novel therapies to lower cardiovascular disease risk
Abstract
Purpose of review: This article summarizes recent observations on the role of lipoprotein(a) [Lp(a)] as a risk factor mediating cardiovascular disease.
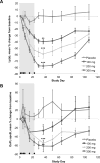
Recent findings: Lp(a) is a highly prevalent cardiovascular risk factor, with levels above 30 mg/dl affecting 20-30% of the global population. Up until now, no specific therapies have been developed to lower Lp(a) levels. Three major levels of evidence support the notion that elevated Lp(a) levels are a causal, independent, genetic risk factor for cardiovascular disease: epidemiologic studies and meta-analyses, genome-wide association studies and Mendelian randomization studies. Recent studies also have noted that individuals with low levels of Lp(a) are associated with a higher risk of incident type 2 diabetes mellitus, and conversely individuals with high levels have a lower risk, but this association does not appear to be causal. Novel therapies to lower Lp(a) include PCSK9 inhibitors and antisense oligonucleotides directly preventing translation of apolipoprotein(a) mRNA.
Summary: With this robust and expanding clinical database, a reawakening of interest in Lp(a) as clinical risk factor is taking place. Trials are underway with novel drugs that substantially lower Lp(a) and may reduce its contribution to cardiovascular disease.
Conflict of interest statement
Dr. Tsimikas is a co-inventor of and receives royalties from patents owned by the University of California San Diego on oxidation-specific antibodies and has a dual appointment at UCSD and Ionis Pharmaceuticals, Inc.
Figures


References
-
- White J, Varvel S, Tsimikas S. Abstract 14669: Prevalence of elevated Lp(a) Levels in 629,858 subjects from a referral laboratory population in the United States. Circulation. 2015;132:A14669.
-
- Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–184. - PubMed
-
- Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

