Exercise training improves vascular mitochondrial function
- PMID: 26825520
- PMCID: PMC4867356
- DOI: 10.1152/ajpheart.00751.2015
Exercise training improves vascular mitochondrial function
Abstract
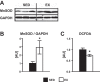
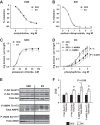
Exercise training is recognized to improve cardiac and skeletal muscle mitochondrial respiratory capacity; however, the impact of chronic exercise on vascular mitochondrial respiratory function is unknown. We hypothesized that exercise training concomitantly increases both vascular mitochondrial respiratory capacity and vascular function. Arteries from both sedentary (SED) and swim-trained (EX, 5 wk) mice were compared in terms of mitochondrial respiratory function, mitochondrial content, markers of mitochondrial biogenesis, redox balance, nitric oxide (NO) signaling, and vessel function. Mitochondrial complex I and complex I + II state 3 respiration and the respiratory control ratio (complex I + II state 3 respiration/complex I state 2 respiration) were greater in vessels from EX relative to SED mice, despite similar levels of arterial citrate synthase activity and mitochondrial DNA content. Furthermore, compared with the SED mice, arteries from EX mice displayed elevated transcript levels of peroxisome proliferative activated receptor-γ coactivator-1α and the downstream targets cytochrome c oxidase subunit IV isoform 1,isocitrate dehydrogenase(Idh)2, and Idh3a, increased manganese superoxide dismutase protein expression, increased endothelial NO synthase phosphorylation (Ser(1177)), and suppressed reactive oxygen species generation (all P< 0.05). Although there were no differences in EX and SED mice concerning endothelium-dependent and endothelium-independent vasorelaxation, phenylephrine-induced vasocontraction was blunted in vessels from EX compared with SED mice, and this effect was normalized by NOS inhibition. These training-induced increases in vascular mitochondrial respiratory capacity and evidence of improved redox balance, which may, at least in part, be attributable to elevated NO bioavailability, have the potential to protect against age- and disease-related challenges to arterial function.
Keywords: arterial function; mitochondria; redox balance; vasculature.
Figures

References
-
- Barbosa VA, Luciano TF, Marques SO, Vitto MF, Souza DR, Silva LA, Santos JP, Moreira JC, Dal-Pizzol F, Lira FS, Pinho RA, De Souza CT. Acute exercise induce endothelial nitric oxide synthase phosphorylation via Akt and AMP-activated protein kinase in aorta of rats: role of reactive oxygen species. Int J Cardiol 167: 2983–2988, 2013. - PubMed
-
- Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol 92: 605–612, 2014. - PMC - PubMed
-
- Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PV, Holland WL, Zhang QJ, Abel ED, Symons JD. Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes 64: 3914–3926, 2015. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

