Dynamin-Related Protein 1 Deficiency Improves Mitochondrial Fitness and Protects against Progression of Diabetic Nephropathy
- PMID: 26825530
- PMCID: PMC5004662
- DOI: 10.1681/ASN.2015101096
Dynamin-Related Protein 1 Deficiency Improves Mitochondrial Fitness and Protects against Progression of Diabetic Nephropathy
Abstract
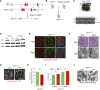
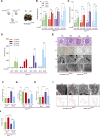
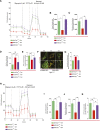
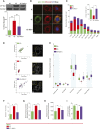
Mitochondrial fission has been linked to the pathogenesis of diabetic nephropathy (DN). However, how mitochondrial fission affects progression of DN in vivo is unknown. Here, we report the effect of conditional podocyte-specific deletion of dynamin-related protein 1 (Drp1), an essential component of mitochondrial fission, on the pathogenesis and progression of DN. Inducible podocyte-specific deletion of Drp1 in diabetic mice decreased albuminuria and improved mesangial matrix expansion and podocyte morphology. Ultrastructure analysis revealed a significant increase in fragmented mitochondria in the podocytes of wild-type diabetic mice but a marked improvement in mitochondrial structure in Drp1-null podocytes of diabetic mice. When isolated from diabetic mice and cultured in high glucose, Drp1-null podocytes had more elongated mitochondria and better mitochondrial fitness associated with enhanced oxygen consumption and ATP production than wild-type podocytes. Furthermore, administration of a pharmacologic inhibitor of Drp1, Mdivi1, significantly blunted mitochondrial fission and rescued key pathologic features of DN in mice. Taken together, these results provide novel correlations between mitochondrial morphology and the progression of DN and point to Drp1 as a potential therapeutic target in DN.
Keywords: diabetic nephropathy; mitochondria; podocyte.
Copyright © 2016 by the American Society of Nephrology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

