Distinct Requirements for Vacuolar Protein Sorting 34 Downstream Effector Phosphatidylinositol 3-Phosphate 5-Kinase in Podocytes Versus Proximal Tubular Cells
- PMID: 26825532
- PMCID: PMC5004647
- DOI: 10.1681/ASN.2015050555
Distinct Requirements for Vacuolar Protein Sorting 34 Downstream Effector Phosphatidylinositol 3-Phosphate 5-Kinase in Podocytes Versus Proximal Tubular Cells
Abstract
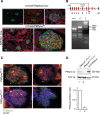
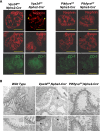
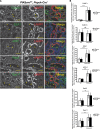
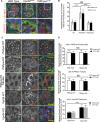
The mechanisms by which the glomerular filtration barrier prevents the loss of large macromolecules and simultaneously, maintains the filter remain poorly understood. Recent studies proposed that podocytes have an active role in both the endocytosis of filtered macromolecules and the maintenance of the filtration barrier. Deletion of a key endosomal trafficking regulator, the class 3 phosphatidylinositol (PtdIns) 3-kinase vacuolar protein sorting 34 (Vps34), in podocytes results in aberrant endosomal membrane morphology and podocyte dysfunction. We recently showed that the vacuolation phenotype in cultured Vps34-deficient podocytes is caused by the absence of a substrate for the Vps34 downstream effector PtdIns 3-phosphate 5-kinase (PIKfyve), which phosphorylates Vps34-generated PtdIns(3)P to produce PtdIns (3,5)P2. PIKfyve perturbation and PtdIns(3,5)P2 reduction result in massive membrane vacuolation along the endosomal system, but the cell-specific functions of PIKfyve in vivo remain unclear. We show here that the genetic deletion of PIKfyve in endocytically active proximal tubular cells resulted in the development of large cytoplasmic vacuoles caused by arrested endocytic traffic progression at a late-endosome stage. In contrast, deletion of PIKfyve in glomerular podocytes did not significantly alter the endosomal morphology, even in age 18-month-old mice. However, on culturing, the PIKfyve-deleted podocytes developed massive cytoplasmic vacuoles. In summary, these data suggest that glomerular podocytes and proximal tubules have different requirements for PIKfyve function, likely related to distinct in vivo needs for endocytic flux.
Keywords: glomerulus; podocyte; proteinuria.
Copyright © 2016 by the American Society of Nephrology.
Figures




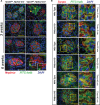
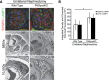
Similar articles
-
Active vacuolar H+ ATPase and functional cycle of Rab5 are required for the vacuolation defect triggered by PtdIns(3,5)P2 loss under PIKfyve or Vps34 deficiency.Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C366-77. doi: 10.1152/ajpcell.00104.2016. Epub 2016 Jun 22. Am J Physiol Cell Physiol. 2016. PMID: 27335171 Free PMC article.
-
Class III PI 3-kinase is the main source of PtdIns3P substrate and membrane recruitment signal for PIKfyve constitutive function in podocyte endomembrane homeostasis.Biochim Biophys Acta. 2015 May;1853(5):1240-50. doi: 10.1016/j.bbamcr.2015.01.008. Epub 2015 Jan 22. Biochim Biophys Acta. 2015. PMID: 25619930 Free PMC article.
-
Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis.J Am Soc Nephrol. 2013 Apr;24(5):727-43. doi: 10.1681/ASN.2012070700. Epub 2013 Mar 14. J Am Soc Nephrol. 2013. PMID: 23492732 Free PMC article.
-
PIKfyve: Partners, significance, debates and paradoxes.Cell Biol Int. 2008 Jun;32(6):591-604. doi: 10.1016/j.cellbi.2008.01.006. Epub 2008 Jan 25. Cell Biol Int. 2008. PMID: 18304842 Free PMC article. Review.
-
Class III phosphatidylinositol 3-kinase and its catalytic product PtdIns3P in regulation of endocytic membrane traffic.FEBS J. 2013 Jun;280(12):2730-42. doi: 10.1111/febs.12116. Epub 2013 Feb 6. FEBS J. 2013. PMID: 23289851 Review.
Cited by
-
Apilimod alters TGFβ signaling pathway and prevents cardiac fibrotic remodeling.Theranostics. 2021 Apr 19;11(13):6491-6506. doi: 10.7150/thno.55821. eCollection 2021. Theranostics. 2021. PMID: 33995670 Free PMC article.
-
Active vacuolar H+ ATPase and functional cycle of Rab5 are required for the vacuolation defect triggered by PtdIns(3,5)P2 loss under PIKfyve or Vps34 deficiency.Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C366-77. doi: 10.1152/ajpcell.00104.2016. Epub 2016 Jun 22. Am J Physiol Cell Physiol. 2016. PMID: 27335171 Free PMC article.
-
PIKfyve Deficiency in Myeloid Cells Impairs Lysosomal Homeostasis in Macrophages and Promotes Systemic Inflammation in Mice.Mol Cell Biol. 2019 Oct 11;39(21):e00158-19. doi: 10.1128/MCB.00158-19. Print 2019 Nov 1. Mol Cell Biol. 2019. PMID: 31427458 Free PMC article.
-
Podocyte Endocytosis in Regulating the Glomerular Filtration Barrier.Front Med (Lausanne). 2022 Feb 10;9:801837. doi: 10.3389/fmed.2022.801837. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35223901 Free PMC article. Review.
-
Role of FGF and Hyaluronan in Choroidal Neovascularization in Sorsby Fundus Dystrophy.Cells. 2020 Mar 4;9(3):608. doi: 10.3390/cells9030608. Cells. 2020. PMID: 32143276 Free PMC article.
References
-
- van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 - PubMed
-
- Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 - PMC - PubMed
-
- Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G: Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int 53: 1209–1216, 1998 - PubMed
-
- Russo LM, Sandoval RM, Brown D, Molitoris BA, Comper WD: Controversies in nephrology: Response to ‘renal albumin handling, facts, and artifacts’. Kidney Int 72: 1195–1197, 2007 - PubMed
-
- Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

