Ferritins: furnishing proteins with iron
- PMID: 26825805
- PMCID: PMC4771812
- DOI: 10.1007/s00775-016-1336-0
Ferritins: furnishing proteins with iron
Abstract
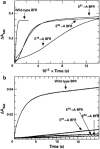
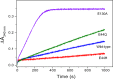
Ferritins are a superfamily of iron oxidation, storage and mineralization proteins found throughout the animal, plant, and microbial kingdoms. The majority of ferritins consist of 24 subunits that individually fold into 4-α-helix bundles and assemble in a highly symmetric manner to form an approximately spherical protein coat around a central cavity into which an iron-containing mineral can be formed. Channels through the coat at inter-subunit contact points facilitate passage of iron ions to and from the central cavity, and intrasubunit catalytic sites, called ferroxidase centers, drive Fe(2+) oxidation and O2 reduction. Though the different members of the superfamily share a common structure, there is often little amino acid sequence identity between them. Even where there is a high degree of sequence identity between two ferritins there can be major differences in how the proteins handle iron. In this review we describe some of the important structural features of ferritins and their mineralized iron cores, consider how iron might be released from ferritins, and examine in detail how three selected ferritins oxidise Fe(2+) to explore the mechanistic variations that exist amongst ferritins. We suggest that the mechanistic differences reflect differing evolutionary pressures on amino acid sequences, and that these differing pressures are a consequence of different primary functions for different ferritins.
Keywords: Bacterioferritin; Ferritin; Ferroxidase; Iron oxidation; Iron storage.
Figures
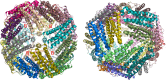




References
-
- Laufberger V. Bull Soc Chim biol. 1937;19:1575–1582.
-
- Lewis CT, Short C (1879) A latin dictionary. Clarendon Press, Oxford. http://www.perseus.tufts.edu. Accessed 3 Jan 2016
-
- Lewin A, Moore GR, Le Brun NE (2005) Dalton Trans, pp 3597–3610 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

