Pregnancy as a Window to Future Cardiovascular Health: Design and Implementation of the nuMoM2b Heart Health Study
- PMID: 26825925
- PMCID: PMC4782765
- DOI: 10.1093/aje/kwv309
Pregnancy as a Window to Future Cardiovascular Health: Design and Implementation of the nuMoM2b Heart Health Study
Abstract
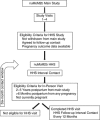
The National Institute of Child Health and Human Development's Nulliparous Pregnancy Outcomes Study-Monitoring Mothers-to-Be (nuMoM2b) Heart Health Study (HHS) was designed to investigate the relationships between adverse pregnancy outcomes and modifiable risk factors for cardiovascular disease. The ongoing nuMoM2b-HHS, which started in 2013, is a prospective follow-up of the nuMoM2b cohort, which included 10,038 women recruited between 2010 and 2013 from 8 centers across the United States who were initially observed over the course of their first pregnancies. In this report, we detail the design and study procedures of the nuMoM2b-HHS. Women in the pregnancy cohort who consented to be contacted for participation in future studies were approached at 6-month intervals to ascertain health information and to maintain ongoing contact. Two to 5 years after completion of the pregnancy documented in the nuMoM2b, women in the nuMoM2b-HHS were invited to an in-person study visit. During this visit, they completed psychosocial and medical history questionnaires and had clinical measurements and biological specimens obtained. A subcohort of participants who had objective assessments of sleep-disordered breathing during pregnancy were asked to repeat this investigation. This unique prospective observational study includes a large, geographically and ethnically diverse cohort, rich depth of phenotypic information about adverse pregnancy outcomes, and clinical data and biospecimens from early in the index pregnancy onward. Data obtained from this cohort will provide mechanistic and clinical insights into how data on a first pregnancy can provide information about the potential development of subsequent risk factors for cardiovascular disease.
Trial registration: ClinicalTrials.gov NCT02231398.
Keywords: cardiovascular disease; cohort study; heart health; methods; pregnancy; women's health.
© The Author 2016. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Rosamond W, Flegal K, Friday G et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;1155:e69–e171. - PubMed
-
- Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;1314:e29–e322. - PubMed
-
- Folsom AR, Kaye SA, Sellers TA et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;2694:483–487. - PubMed
-
- Gordon T, Castelli WP, Hjortland MC et al. Diabetes, blood lipids, and the role of obesity in coronary heart disease risk for women. The Framingham Study. Ann Intern Med. 1977;874:393–397. - PubMed
-
- Manson JE, Colditz GA, Stampfer MJ et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;32213:882–889. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U10 HL120018/HL/NHLBI NIH HHS/United States
- UL1TR001108/TR/NCATS NIH HHS/United States
- U10-HL119990/HL/NHLBI NIH HHS/United States
- U10-HL120018/HL/NHLBI NIH HHS/United States
- UL1TR000153/TR/NCATS NIH HHS/United States
- UL1 TR000153/TR/NCATS NIH HHS/United States
- U10 HL119993/HL/NHLBI NIH HHS/United States
- U10 HL119992/HL/NHLBI NIH HHS/United States
- U10-HL119991/HL/NHLBI NIH HHS/United States
- U10 HL120034/HL/NHLBI NIH HHS/United States
- UL1 TR001067/TR/NCATS NIH HHS/United States
- U10 HL120006/HL/NHLBI NIH HHS/United States
- U10-HL119989/HL/NHLBI NIH HHS/United States
- U10-HL119992/HL/NHLBI NIH HHS/United States
- U10 HL119991/HL/NHLBI NIH HHS/United States
- U10 HD063036/HD/NICHD NIH HHS/United States
- U10 HL119989/HL/NHLBI NIH HHS/United States
- U10 HL119990/HL/NHLBI NIH HHS/United States
- U10-HL119993/HL/NHLBI NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- U10-HL120019/HL/NHLBI NIH HHS/United States
- U10-HL120006/HL/NHLBI NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- P2C HD047873/HD/NICHD NIH HHS/United States
- U10-HL120034/HL/NHLBI NIH HHS/United States
- U10 HL120019/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical