Differential Roles of Protein Complexes NOX1-NOXO1 and NOX2-p47phox in Mediating Endothelial Redox Responses to Oscillatory and Unidirectional Laminar Shear Stress
- PMID: 26826128
- PMCID: PMC4861435
- DOI: 10.1074/jbc.M115.713149
Differential Roles of Protein Complexes NOX1-NOXO1 and NOX2-p47phox in Mediating Endothelial Redox Responses to Oscillatory and Unidirectional Laminar Shear Stress
Abstract
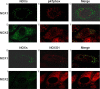
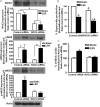
The endothelium is exposed to various flow patterns such as vasoprotective unidirectional laminar shear stress (LSS) and atherogenic oscillatory shear stress (OSS). A software-controlled, valve-operated OsciFlow device with parallel chambers was used to apply LSS and OSS to endothelial cells. Although LSS inhibited superoxide over time, OSS time-dependently increased superoxide production from endothelial cells. Immunocytochemical staining revealed that, at resting state, p47phox colocalizes with NOX2, whereas NOXO1 colocalizes with NOX1. RNAi of p47phox had no effects on superoxide or NO production in response to OSS but significantly reduced NO production in LSS, implicating a p47phox-bound NADPH oxidase (NOX) in mediating basal NO production. Indeed, RNAi of p47phox inhibited endothelial nitric oxide synthase (eNOS) serine 1179 phosphorylation, whereas PEG-catalase scavenging of intracellular hydrogen peroxide or RNAi of NOX2 produced similar results, indicating a role of NOX2/p47phox-derived hydrogen peroxide in mediating the basal activity of NO production from eNOS. In contrast, RNAi of NOXO1 resulted in no significant changes in NO and superoxide levels in response to LSS but significantly reduced superoxide while increasing NO in response to OSS. Furthermore, we identified, for the first time, that OSS uncouples eNOS, which was corrected by RNAi of NOXO1. In summary, LSS activates the NOX2-p47phox complex to activate eNOS phosphorylation and NO production. OSS instead activates the NOX1-NOXO1 complex to uncouple eNOS. These results demonstrate differential roles of NOXs in modulating the redox state in response to different shear stresses, which may promote the development of novel therapeutic agents to mimic the protective effects of LSS while inhibiting the injurious effects of OSS.
Keywords: NADPH oxidase; nitric oxide; nitric oxide synthase; oxidative stress; shear stress; signal transduction.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







Similar articles
-
Oscillatory shear stress upregulation of endothelial nitric oxide synthase requires intracellular hydrogen peroxide and CaMKII.J Mol Cell Cardiol. 2004 Jul;37(1):121-5. doi: 10.1016/j.yjmcc.2004.04.012. J Mol Cell Cardiol. 2004. PMID: 15242742
-
The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes.Diabetologia. 2012 Jul;55(7):2069-79. doi: 10.1007/s00125-012-2557-6. Epub 2012 May 2. Diabetologia. 2012. PMID: 22549734 Free PMC article.
-
NOX4-dependent Hydrogen peroxide promotes shear stress-induced SHP2 sulfenylation and eNOS activation.Free Radic Biol Med. 2015 Dec;89:419-30. doi: 10.1016/j.freeradbiomed.2015.08.014. Epub 2015 Sep 30. Free Radic Biol Med. 2015. PMID: 26427883
-
Organizers and activators: Cytosolic Nox proteins impacting on vascular function.Free Radic Biol Med. 2017 Aug;109:22-32. doi: 10.1016/j.freeradbiomed.2017.03.017. Epub 2017 Mar 21. Free Radic Biol Med. 2017. PMID: 28336130 Review.
-
Luminal flow regulates NO and O2(-) along the nephron.Am J Physiol Renal Physiol. 2011 May;300(5):F1047-53. doi: 10.1152/ajprenal.00724.2010. Epub 2011 Feb 23. Am J Physiol Renal Physiol. 2011. PMID: 21345976 Free PMC article. Review.
Cited by
-
Mitochondrial Ca2+ transport in the endothelium: regulation by ions, redox signalling and mechanical forces.J R Soc Interface. 2017 Dec;14(137):20170672. doi: 10.1098/rsif.2017.0672. Epub 2017 Dec 13. J R Soc Interface. 2017. PMID: 29237825 Free PMC article. Review.
-
Microvascular NADPH oxidase in health and disease.Free Radic Biol Med. 2017 Aug;109:33-47. doi: 10.1016/j.freeradbiomed.2017.02.049. Epub 2017 Mar 6. Free Radic Biol Med. 2017. PMID: 28274817 Free PMC article. Review.
-
Interplay between energy metabolism and NADPH oxidase-mediated pathophysiology in cardiovascular diseases.Front Pharmacol. 2025 Jan 10;15:1503824. doi: 10.3389/fphar.2024.1503824. eCollection 2024. Front Pharmacol. 2025. PMID: 39867658 Free PMC article. Review.
-
Multiple sclerosis: molecular mechanisms and therapeutic opportunities.Antioxid Redox Signal. 2013 Dec 20;19(18):2286-334. doi: 10.1089/ars.2012.5068. Epub 2013 Apr 22. Antioxid Redox Signal. 2013. PMID: 23473637 Free PMC article. Review.
-
Amelioration of endothelial dysfunction by sodium glucose co-transporter 2 inhibitors: pieces of the puzzle explaining their cardiovascular protection.Br J Pharmacol. 2022 Aug;179(16):4047-4062. doi: 10.1111/bph.15850. Epub 2022 Apr 22. Br J Pharmacol. 2022. PMID: 35393687 Free PMC article. Review.
References
-
- Davis M. E., Cai H., Drummond G. R., and Harrison D. G. (2001) Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ. Res. 89, 1073–1080 - PubMed
-
- Wang J., Wolin M. S., and Hintze T. H. (1993) Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ. Res. 73, 829–838 - PubMed
-
- Hwang J., Saha A., Boo Y. C., Sorescu G. P., McNally J. S., Holland S. M., Dikalov S., Giddens D. P., Griendling K. K., Harrison D. G., and Jo H. (2003) Oscillatory shear stress stimulates endothelial production of O2˙̄ from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J. Biol. Chem. 278, 47291–47298 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

