Core pathway mutations induce de-differentiation of murine astrocytes into glioblastoma stem cells that are sensitive to radiation but resistant to temozolomide
- PMID: 26826202
- PMCID: PMC4896545
- DOI: 10.1093/neuonc/nov321
Core pathway mutations induce de-differentiation of murine astrocytes into glioblastoma stem cells that are sensitive to radiation but resistant to temozolomide
Abstract
Background: Glioma stem cells (GSCs) from human glioblastomas (GBMs) are resistant to radiation and chemotherapy and may drive recurrence. Treatment efficacy may depend on GSCs, expression of DNA repair enzymes such as methylguanine methyltransferase (MGMT), or transcriptome subtype.
Methods: To model genetic alterations in human GBM core signaling pathways, we induced Rb knockout, Kras activation, and Pten deletion mutations in cortical murine astrocytes. Neurosphere culture, differentiation, and orthotopic transplantation assays were used to assess whether these mutations induced de-differentiation into GSCs. Genome-wide chromatin landscape alterations and expression profiles were examined by formaldehyde-assisted isolation of regulatory elements (FAIRE) seq and RNA-seq. Radiation and temozolomide efficacy were examined in vitro and in an allograft model in vivo. Effects of radiation on transcriptome subtype were examined by microarray expression profiling.
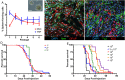
Results: Cultured triple mutant astrocytes gained unlimited self-renewal and multilineage differentiation capacity. These cells harbored significantly altered chromatin landscapes that were associated with downregulation of astrocyte- and upregulation of stem cell-associated genes, particularly the Hoxa locus of embryonic transcription factors. Triple-mutant astrocytes formed serially transplantable glioblastoma allografts that were sensitive to radiation but expressed MGMT and were resistant to temozolomide. Radiation induced a shift in transcriptome subtype of GBM allografts from proneural to mesenchymal.
Conclusion: A defined set of core signaling pathway mutations induces de-differentiation of cortical murine astrocytes into GSCs with altered chromatin landscapes and transcriptomes. This non-germline genetically engineered mouse model mimics human proneural GBM on histopathological, molecular, and treatment response levels. It may be useful for dissecting the mechanisms of treatment resistance and developing more effective therapies.
Keywords: Astrocytes; glioblastoma; glioma stem cells; radiation; temozolomide.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Similar articles
-
The histone demethylase KDM5A is a key factor for the resistance to temozolomide in glioblastoma.Cell Cycle. 2015;14(21):3418-29. doi: 10.1080/15384101.2015.1090063. Cell Cycle. 2015. PMID: 26566863 Free PMC article.
-
Dual mTORC1/2 blockade inhibits glioblastoma brain tumor initiating cells in vitro and in vivo and synergizes with temozolomide to increase orthotopic xenograft survival.Clin Cancer Res. 2014 Nov 15;20(22):5756-67. doi: 10.1158/1078-0432.CCR-13-3389. Epub 2014 Oct 14. Clin Cancer Res. 2014. PMID: 25316808
-
Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization.Neuro Oncol. 2018 Jan 22;20(2):236-248. doi: 10.1093/neuonc/nox142. Neuro Oncol. 2018. PMID: 29016925 Free PMC article.
-
Chemoresistance of glioblastoma cancer stem cells--much more complex than expected.Mol Cancer. 2011 Oct 11;10:128. doi: 10.1186/1476-4598-10-128. Mol Cancer. 2011. PMID: 21988793 Free PMC article. Review.
-
Cancer Stem Cells and Chemoresistance in Glioblastoma Multiform: A Review Article.J Stem Cells. 2015;10(4):271-85. J Stem Cells. 2015. PMID: 27144829 Review.
Cited by
-
Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model.Neuro Oncol. 2016 Dec;18(12):1622-1633. doi: 10.1093/neuonc/now117. Epub 2016 Jun 13. Neuro Oncol. 2016. PMID: 27298311 Free PMC article.
-
PIK3CA missense mutations promote glioblastoma pathogenesis, but do not enhance targeted PI3K inhibition.PLoS One. 2018 Jul 5;13(7):e0200014. doi: 10.1371/journal.pone.0200014. eCollection 2018. PLoS One. 2018. PMID: 29975751 Free PMC article.
-
Neuronal activity modifies the chromatin accessibility landscape in the adult brain.Nat Neurosci. 2017 Mar;20(3):476-483. doi: 10.1038/nn.4494. Epub 2017 Feb 6. Nat Neurosci. 2017. PMID: 28166220 Free PMC article.
-
Increased HOXA5 expression provides a selective advantage for gain of whole chromosome 7 in IDH wild-type glioblastoma.Genes Dev. 2018 Apr 1;32(7-8):512-523. doi: 10.1101/gad.312157.118. Epub 2018 Apr 9. Genes Dev. 2018. PMID: 29632085 Free PMC article.
-
Evaluating glioblastoma tumour sphere growth and migration in interaction with astrocytes using 3D collagen-hyaluronic acid hydrogels.J Mater Chem B. 2023 Jun 21;11(24):5442-5459. doi: 10.1039/d3tb00066d. J Mater Chem B. 2023. PMID: 37159233 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Galli R, Binda E, Orfanelli U et al. . Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. - PubMed
-
- Singh SK, Hawkins C, Clarke ID et al. . Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. - PubMed
-
- Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6(6):425–436. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

