A Highly Arginolytic Streptococcus Species That Potently Antagonizes Streptococcus mutans
- PMID: 26826230
- PMCID: PMC4807514
- DOI: 10.1128/AEM.03887-15
A Highly Arginolytic Streptococcus Species That Potently Antagonizes Streptococcus mutans
Abstract
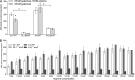
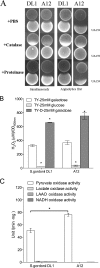
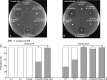
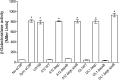
The ability of certain oral biofilm bacteria to moderate pH through arginine metabolism by the arginine deiminase system (ADS) is a deterrent to the development of dental caries. Here, we characterize a novel Streptococcus strain, designated strain A12, isolated from supragingival dental plaque of a caries-free individual. A12 not only expressed the ADS pathway at high levels under a variety of conditions but also effectively inhibited growth and two intercellular signaling pathways of the dental caries pathogen Streptococcus mutans. A12 produced copious amounts of H2O2 via the pyruvate oxidase enzyme that were sufficient to arrest the growth of S. mutans. A12 also produced a protease similar to challisin (Sgc) of Streptococcus gordonii that was able to block the competence-stimulating peptide (CSP)-ComDE signaling system, which is essential for bacteriocin production by S. mutans. Wild-type A12, but not an sgc mutant derivative, could protect the sensitive indicator strain Streptococcus sanguinis SK150 from killing by the bacteriocins of S. mutans. A12, but not S. gordonii, could also block the XIP (comX-inducing peptide) signaling pathway, which is the proximal regulator of genetic competence in S. mutans, but Sgc was not required for this activity. The complete genome sequence of A12 was determined, and phylogenomic analyses compared A12 to streptococcal reference genomes. A12 was most similar to Streptococcus australis and Streptococcus parasanguinis but sufficiently different that it may represent a new species. A12-like organisms may play crucial roles in the promotion of stable, health-associated oral biofilm communities by moderating plaque pH and interfering with the growth and virulence of caries pathogens.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Novel Probiotic Mechanisms of the Oral Bacterium Streptococcus sp. A12 as Explored with Functional Genomics.Appl Environ Microbiol. 2019 Oct 16;85(21):e01335-19. doi: 10.1128/AEM.01335-19. Print 2019 Nov 1. Appl Environ Microbiol. 2019. PMID: 31420345 Free PMC article.
-
Interactions between oral bacteria: inhibition of Streptococcus mutans bacteriocin production by Streptococcus gordonii.Appl Environ Microbiol. 2005 Jan;71(1):354-62. doi: 10.1128/AEM.71.1.354-362.2005. Appl Environ Microbiol. 2005. PMID: 15640209 Free PMC article.
-
Diversity in Antagonistic Interactions between Commensal Oral Streptococci and Streptococcus mutans.Caries Res. 2018;52(1-2):88-101. doi: 10.1159/000479091. Epub 2017 Dec 20. Caries Res. 2018. PMID: 29258070 Free PMC article.
-
[Streptococcus mutans and oral streptococci in dental plaque].Can J Microbiol. 2011 Jan;57(1):1-20. doi: 10.1139/w10-095. Can J Microbiol. 2011. PMID: 21217792 Review. French.
-
Quorum sensing and biofilm formation by Streptococcus mutans.Adv Exp Med Biol. 2008;631:178-88. doi: 10.1007/978-0-387-78885-2_12. Adv Exp Med Biol. 2008. PMID: 18792689 Review.
Cited by
-
Presence of Streptococcus dentisani in the dental plaque of children from different Colombian cities.Clin Exp Dent Res. 2019 May 9;5(3):184-190. doi: 10.1002/cre2.158. eCollection 2019 Jun. Clin Exp Dent Res. 2019. PMID: 31249697 Free PMC article.
-
The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems.Microorganisms. 2020 Feb 23;8(2):308. doi: 10.3390/microorganisms8020308. Microorganisms. 2020. PMID: 32102216 Free PMC article. Review.
-
[Association of sepM gene mutation with mutacin Ⅳ production by Streptococcus mutans].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Jun 20;41(6):876-882. doi: 10.12122/j.issn.1673-4254.2021.06.10. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34238740 Free PMC article. Chinese.
-
Microbiota transplantation.Heliyon. 2024 Oct 11;10(20):e39047. doi: 10.1016/j.heliyon.2024.e39047. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640634 Free PMC article. Review.
-
Insight into the Effect of Small RNA srn225147 on Mutacin IV in Streptococcus mutans.Indian J Microbiol. 2019 Dec;59(4):445-450. doi: 10.1007/s12088-019-00820-2. Epub 2019 Aug 28. Indian J Microbiol. 2019. PMID: 31762507 Free PMC article.
References
-
- Huang X, Guo Q, Ren B, Li Y, Zhou X. 2016. Models in caries research, p 157–173. In Zhou X. (ed), Dental caries. Springer, Berlin, Germany.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

