Enhancement of Microbiota in Healthy Macaques Results in Beneficial Modulation of Mucosal and Systemic Immune Function
- PMID: 26826246
- PMCID: PMC4761491
- DOI: 10.4049/jimmunol.1502470
Enhancement of Microbiota in Healthy Macaques Results in Beneficial Modulation of Mucosal and Systemic Immune Function
Abstract
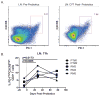
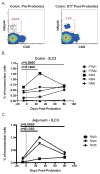
Given the critical role of mucosal surfaces in susceptibility to infection, it is imperative that effective mucosal responses are induced when developing efficacious vaccines and prevention strategies for infection. Modulating the microbiota in the gastrointestinal (GI) tract through the use of probiotics (PBio) is a safe and well-tolerated approach to enhance mucosal and overall health. We assessed the longitudinal impact of daily treatment with the VSL#3 probiotic on cellular and humoral immunity and inflammation in healthy macaques. PBio therapy resulted in significantly increased frequencies of B cells expressing IgA in the colon and lymph node (LN), likely because of significantly increased LN T follicular helper cell frequencies and LN follicles. Increased frequencies of IL-23(+) APCs in the colon were found post-PBio treatment, which correlated with LN T follicular helper cells. Finally, VSL#3 significantly downmodulated the response of TLR2-, TLR3-, TLR4-, and TLR9-expressing HEK293 cells to stimulation with Pam3CSK4, polyinosinic-polycytidylic acid, LPS, and ODN2006, respectively. These data provide a mechanism for the beneficial impact of PBio on mucosal health and implicates the use of PBio therapy in the context of vaccination or preventative approaches to enhance protection from mucosal infection by improving immune defenses at the mucosal portal of entry.
Copyright © 2016 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, Rehakova Z, Sinkora J, Hofman J, Drastich P, Kokesova A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology letters. 2004;93:97–108. - PubMed
-
- Kaushic C. The role of the local microenvironment in regulating susceptibility and immune responses to sexually transmitted viruses in the female genital tract. Journal of reproductive immunology. 2009;83:168–172. - PubMed
-
- Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Current opinion in immunology. 2015;36:22–30. - PubMed
-
- Reeves RK, Burgener A, Klatt NR. Targeting the gastrointestinal tract to develop novel therapies for HIV. Clinical pharmacology and therapeutics. 2015;98:381–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

