CD4 Cell Count: Declining Value for Antiretroviral Therapy Eligibility
- PMID: 26826372
- PMCID: PMC5006297
- DOI: 10.1093/cid/civ1224
CD4 Cell Count: Declining Value for Antiretroviral Therapy Eligibility
Erratum in
-
Ying R, et al (Clin Infect Dis 2016; 62:1022-8).Clin Infect Dis. 2016 Jun 15;62(12):1621. doi: 10.1093/cid/ciw268. Epub 2016 May 3. Clin Infect Dis. 2016. PMID: 27143658 Free PMC article. No abstract available.
Abstract
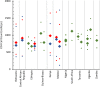
Antiretroviral therapy (ART) policy for people living with human immunodeficiency virus (HIV) has historically been based on clinical indications, such as opportunistic infections and CD4 cell counts. Studies suggest that CD4 counts early in HIV infection do not predict relevant public health outcomes such as disease progression, mortality, and HIV transmission in people living with HIV. CD4 counts also vary widely within individuals and among populations, leading to imprecise measurements and arbitrary ART initiation. To capture the clinical and preventive benefits of treatment, the global HIV response now focuses on increasing HIV diagnosis and ART coverage. CD4 counts for ART initiation were necessary when medications were expensive and had severe side effects, and when the impact of early ART initiation was unclear. However, current evidence suggests that although CD4 counts may still play a role in guiding clinical care to start prophylaxis for opportunistic infections, CD4 counts should cease to be required for ART initiation.
Keywords: ART; CD4 cell count; HIV; care continuum; universal test and treat.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures
References
-
- Hammer SM, Squires KE, Hughes MD et al. . A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997; 337:725–33. - PubMed
-
- Gulick RM, Mellors JW, Havlir D et al. . Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 1997; 337:734–9. - PubMed
-
- Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science 2000; 287:650–4. - PubMed
-
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. - PubMed
-
- Montaner JS, Hogg R, Wood E et al. . The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 2006; 368:531–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

