Increasing tetrahydrobiopterin in cardiomyocytes adversely affects cardiac redox state and mitochondrial function independently of changes in NO production
- PMID: 26826575
- PMCID: PMC5498285
- DOI: 10.1016/j.freeradbiomed.2016.01.019
Increasing tetrahydrobiopterin in cardiomyocytes adversely affects cardiac redox state and mitochondrial function independently of changes in NO production
Abstract
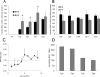
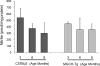
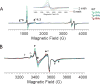
Tetrahydrobiopterin (BH4) represents a potential strategy for the treatment of cardiac remodeling, fibrosis and/or diastolic dysfunction. The effects of oral treatment with BH4 (Sapropterin™ or Kuvan™) are however dose-limiting with high dose negating functional improvements. Cardiomyocyte-specific overexpression of GTP cyclohydrolase I (mGCH) increases BH4 several-fold in the heart. Using this model, we aimed to establish the cardiomyocyte-specific responses to high levels of BH4. Quantification of BH4 and BH2 in mGCH transgenic hearts showed age-based variations in BH4:BH2 ratios. Hearts of mice (<6 months) have lower BH4:BH2 ratios than hearts of older mice while both GTPCH activity and tissue ascorbate levels were higher in hearts of young than older mice. No evident changes in nitric oxide (NO) production assessed by nitrite and endogenous iron-nitrosyl complexes were detected in any of the age groups. Increased BH4 production in cardiomyocytes resulted in a significant loss of mitochondrial function. Diminished oxygen consumption and reserve capacity was verified in mitochondria isolated from hearts of 12-month old compared to 3-month old mice, even though at 12 months an improved BH4:BH2 ratio is established. Accumulation of 4-hydroxynonenal (4-HNE) and decreased glutathione levels were found in the mGCH hearts and isolated mitochondria. Taken together, our results indicate that the ratio of BH4:BH2 does not predict changes in neither NO levels nor cellular redox state in the heart. The BH4 oxidation essentially limits the capacity of cardiomyocytes to reduce oxidant stress. Cardiomyocyte with chronically high levels of BH4 show a significant decline in redox state and mitochondrial function.
Keywords: 7,8-Dihydrobiopterin; Biopterin; Electron paramagnetic resonance; GTP cyclohydrolase I; Neopterin.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Characterization of cerebral microvasculature in transgenic mice with endothelium targeted over-expression of GTP-cyclohydrolase I.Brain Res. 2015 Nov 2;1625:198-205. doi: 10.1016/j.brainres.2015.08.034. Epub 2015 Sep 3. Brain Res. 2015. PMID: 26343845 Free PMC article.
-
Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways.J Biol Chem. 2009 Oct 9;284(41):28128-28136. doi: 10.1074/jbc.M109.041483. Epub 2009 Aug 7. J Biol Chem. 2009. PMID: 19666465 Free PMC article.
-
Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells.J Biol Chem. 2009 May 8;284(19):12691-700. doi: 10.1074/jbc.M809295200. Epub 2009 Mar 12. J Biol Chem. 2009. PMID: 19286667 Free PMC article.
-
Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease.Arterioscler Thromb Vasc Biol. 2004 Mar;24(3):413-20. doi: 10.1161/01.ATV.0000110785.96039.f6. Epub 2003 Dec 4. Arterioscler Thromb Vasc Biol. 2004. PMID: 14656731 Review.
-
Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling.Curr Heart Fail Rep. 2012 Sep;9(3):200-10. doi: 10.1007/s11897-012-0097-5. Curr Heart Fail Rep. 2012. PMID: 22711313 Free PMC article. Review.
Cited by
-
A novel role for endothelial tetrahydrobiopterin in mitochondrial redox balance.Free Radic Biol Med. 2017 Mar;104:214-225. doi: 10.1016/j.freeradbiomed.2017.01.012. Epub 2017 Jan 17. Free Radic Biol Med. 2017. PMID: 28104455 Free PMC article.
-
Microbial metabolites associated in stool and left ventricle of heart failure patients revealed by meta-analysis.Sci Rep. 2025 Apr 25;15(1):14576. doi: 10.1038/s41598-025-96695-z. Sci Rep. 2025. PMID: 40280962 Free PMC article.
-
Transgenic overexpression of GTP cyclohydrolase 1 in cardiomyocytes ameliorates post-infarction cardiac remodeling.Sci Rep. 2017 Jun 8;7(1):3093. doi: 10.1038/s41598-017-03234-6. Sci Rep. 2017. PMID: 28596578 Free PMC article.
-
Low-Temperature EPR Spectroscopy as a Probe-Free Technique for Monitoring Oxidants Formed in Tumor Cells and Tissues: Implications in Drug Resistance and OXPHOS-Targeted Therapies.Cell Biochem Biophys. 2019 Mar;77(1):89-98. doi: 10.1007/s12013-018-0858-1. Epub 2018 Sep 26. Cell Biochem Biophys. 2019. PMID: 30259334 Free PMC article.
-
Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection-Protocol Optimization and Pitfalls.Antioxidants (Basel). 2022 Jun 16;11(6):1182. doi: 10.3390/antiox11061182. Antioxidants (Basel). 2022. PMID: 35740082 Free PMC article.
References
-
- Kwon NS, Nathan CF, Stuehr DJ. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989;264:20496–20501. - PubMed
-
- Mayer B, John M, Heinzel B, Werner ER, Wachter H, Schultz G, Böhme E. Brain nitric oxide synthase is a biopterin- and flavin-containing multi-functional oxido-reductase. FEBS Lett. 1991;288:187–191. - PubMed
-
- Gross SS, Jaffe EA, Levi R, Kilbourn RG. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem Biophys Res Commun. 1991;178:823–829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

