Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis
- PMID: 26826670
- PMCID: PMC4761718
- DOI: 10.18632/aging.100879
Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis
Abstract
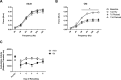
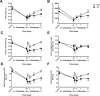
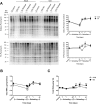
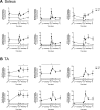
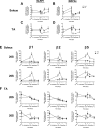
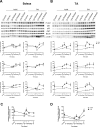
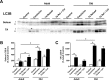
Age-related loss of muscle mass and strength can be accelerated by impaired recovery of muscle mass following a transient atrophic stimulus. The aim of this study was to identify the mechanisms underlying the attenuated recovery of muscle mass and strength in old rats following disuse-induced atrophy. Adult (9 month) and old (29 month) male F344BN rats underwent hindlimb unloading (HU) followed by reloading. HU induced significant atrophy of the hindlimb muscles in both adult (17-38%) and old (8-29%) rats, but only the adult rats exhibited full recovery of muscle mass and strength upon reloading. Upon reloading, total RNA and protein synthesis increased to a similar extent in adult and old muscles. At baseline and upon reloading, however, proteasome-mediated degradation was suppressed leading to an accumulation of ubiquitin-tagged proteins and p62. Further, ER stress, as measured by CHOP expression, was elevated at baseline and upon reloading in old rats. Analysis of mRNA expression revealed increases in HDAC4, Runx1, myogenin, Gadd45a, and the AChRs in old rats, suggesting neuromuscular junction instability/denervation. Collectively, our data suggests that with aging, impaired neuromuscular transmission and deficits in the proteostasis network contribute to defects in muscle fiber remodeling and functional recovery of muscle mass and strength.
Keywords: aging; anabolic resistance; autophagy; hindlimb unloading; ubiquitin proteasome system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. The journals of gerontology Series, A, Biological sciences and medical sciences. 2003;58:M911–916. - PubMed
-
- Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clinical Cancer Research. 2009;15:2920–2926. - PubMed
-
- Montano-Loza AJ. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transplantation. 2014;20:1424–1424. - PubMed
-
- Carey EJ. Sarcopenia in Solid Organ Transplantation. Nutrition in Clinical Practice. 2014;29:159–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

