Addiction-like Synaptic Impairments in Diet-Induced Obesity
- PMID: 26826876
- PMCID: PMC4889544
- DOI: 10.1016/j.biopsych.2015.11.019
Addiction-like Synaptic Impairments in Diet-Induced Obesity
Abstract
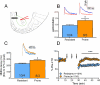
Background: There is increasing evidence that the pathological overeating underlying some forms of obesity is compulsive in nature and therefore contains elements of an addictive disorder. However, direct physiological evidence linking obesity to synaptic plasticity akin to that occurring in addiction is lacking. We sought to establish whether the propensity to diet-induced obesity (DIO) is associated with addictive-like behavior, as well as synaptic impairments in the nucleus accumbens core considered hallmarks of addiction.
Methods: Sprague Dawley rats were allowed free access to a palatable diet for 8 weeks then separated by weight gain into DIO-prone and DIO-resistant subgroups. Access to palatable food was then restricted to daily operant self-administration sessions using fixed ratio 1, 3, and 5 and progressive ratio schedules. Subsequently, nucleus accumbens brain slices were prepared, and we tested for changes in the ratio between α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate currents and the ability to exhibit long-term depression.
Results: We found that propensity to develop DIO is linked to deficits in the ability to induce long-term depression in the nucleus accumbens, as well as increased potentiation at these synapses as measured by AMPA/N-methyl-D-aspartate currents. Consistent with these impairments, we observed addictive-like behavior in DIO-prone rats, including 1) heightened motivation for palatable food; 2) excessive intake; and 3) increased food seeking when food was unavailable.
Conclusions: Our results show overlap between the propensity for DIO and the synaptic changes associated with facets of addictive behavior, supporting partial coincident neurological underpinnings for compulsive overeating and drug addiction.
Keywords: Food addiction; Glutamate; Long-term depression; Nucleus accumbens; Obesity; Synaptic plasticity.
Copyright © 2016 Society of Biological Psychiatry. All rights reserved.
Figures
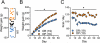




Comment in
-
New Evidence Linking Obesity and Food Addiction.Biol Psychiatry. 2017 May 1;81(9):734-736. doi: 10.1016/j.biopsych.2017.02.1179. Biol Psychiatry. 2017. PMID: 28391804 No abstract available.
References
-
- NIDDK . Network WW-cI. NIDDK; 2005. Overweight and Obesity Statistics.
-
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

