Melphalan-flufenamide is cytotoxic and potentiates treatment with chemotherapy and the Src inhibitor dasatinib in urothelial carcinoma
- PMID: 26827254
- PMCID: PMC5423156
- DOI: 10.1016/j.molonc.2015.12.013
Melphalan-flufenamide is cytotoxic and potentiates treatment with chemotherapy and the Src inhibitor dasatinib in urothelial carcinoma
Abstract
Background: Chemotherapy options in advanced urothelial carcinoma (UC) remain limited. Here we evaluated the peptide-based alkylating agent melphalan-flufenamide (mel-flufen) for UC.
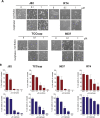
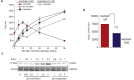
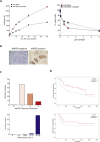
Methods: UC cell lines J82, RT4, TCCsup and 5637 were treated with mel-flufen, alone or combined with cisplatin, gemcitabine, dasatinib or bestatin. Cell viability (MTT assay), intracellular drug accumulation (liquid chromatography) apoptosis induction (apoptotic cell nuclei morphology, western blot analysis of PARP-1/caspase-9 cleavage and Bak/Bax activation) were evaluated. Kinome alterations were characterized by PathScan array and phospho-Src validated by western blotting. Aminopeptidase N (ANPEP) expression was evaluated in UC clinical specimens in relation to patient outcome.
Results: In J82, RT4, TCCsup and 5637 UC cells, mel-flufen amplified the intracellular loading of melphalan in part via aminopeptidase N (ANPEP), resulting in increased cytotoxicity compared to melphalan alone. Mel-flufen induced apoptosis seen as activation of Bak/Bax, cleavage of caspase-9/PARP-1 and induction of apoptotic cell nuclei morphology. Combining mel-flufen with cisplatin or gemcitabine in J82 cells resulted in additive cytotoxic effects and for gemcitabine also increased apoptosis induction. Profiling of mel-flufen-induced kinome alterations in J82 cells revealed that mel-flufen alone did not inhibit Src phosphorylation. Accordingly, the Src inhibitor dasatinib sensitized for mel-flufen cytotoxicity. Immunohistochemical analysis of the putative mel-flufen biomarker ANPEP demonstrated prominent expression levels in tumours from 82 of 83 cystectomy patients. Significantly longer median overall survival was found in patients with high ANPEP expression (P = 0.02).
Conclusion: Mel-flufen alone or in combination with cisplatin, gemcitabine or Src inhibition holds promise as a novel treatment for UC.
Keywords: Aminopeptidase N; Apoptosis; Cisplatin; Dasatinib; Gemcitabine; Melphalan-flufenamide; Src; Urothelial carcinoma.
Copyright © 2016 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Bellmunt, J. , Theodore, C. , Demkov, T. , Komyakov, B. , Sengelov, L. , Daugaard, G. , Caty, A. , Carles, J. , Jagiello-Gruszfeld, A. , Karyakin, O. , Delgado, F.M. , Hurteloup, P. , Winquist, E. , Morsli, N. , Salhi, Y. , Culine, S. , von der Maase, H. , 2009. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 27, 4454–4461. - PubMed
-
- Bellmunt, J. , Fougeray, R. , Rosenberg, J.E. , von der Maase, H. , Schutz, F.A. , Salhi, Y. , Culine, S. , Choueiri, T.K. , 2013. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann. Oncol. 24, 1466–1472. - PubMed
-
- Berglund, Å. , Ullén, A. , Lisyanskaya, A. , Orlov, S. , Hagberg, H. , Tholander, B. , Lewensohn, R. , Nygren, P. , Spira, J. , Harmenberg, J. , Jerling, M. , Alvfors, C. , Ringbom, M. , Nordström, E. , Söderlind, K. , Gullbo, J. , 2015. First-in-human, phase I/IIa clinical study of the peptidase potentiated alkylator melflufen administered every three weeks to patients with advanced solid tumor malignancies. Invest. New Drugs. 33, 1232–1241. - PubMed
-
- Chauhan, D. , Ray, A. , Viktorsson, K. , Spira, J. , Paba-Prada, C. , Munshi, N. , Richardson, P. , Lewensohn, R. , Anderson, K.C. , 2013. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin. Cancer Res. 19, 3019–3031. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

