IRB reliance: An informatics approach
- PMID: 26827623
- PMCID: PMC4837001
- DOI: 10.1016/j.jbi.2016.01.011
IRB reliance: An informatics approach
Abstract
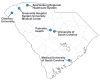
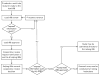
Multi-site Institutional Review Board (IRB) review of clinical research projects is an important but complex and time-consuming activity that is hampered by disparate non-interoperable computer systems for management of IRB applications. This paper describes our work toward harmonizing the workflow and data model of IRB applications through the development of a software-as-a-service shared-IRB platform for five institutions in South Carolina. Several commonalities and differences were recognized across institutions and a core data model that included the data elements necessary for IRB applications across all institutions was identified. We extended and modified the system to support collaborative reviews of IRB proposals within routine workflows of participating IRBs. Overall about 80% of IRB application content was harmonized across all institutions, establishing the foundation for a streamlined cooperative review and reliance. Since going live in 2011, 49 applications that underwent cooperative reviews over a three year period were approved, with the majority involving 2 out of 5 institutions. We believe this effort will inform future work on a common IRB data model that will allow interoperability through a federated approach for sharing IRB reviews and decisions with the goal of promoting reliance across institutions in the translational research community at large.
Keywords: Clinical research; Data model; IRB; Informatics; Reliance; Workflow.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Services USDoHH, editor. Basic HHS policy for protection of human research subjects. Vol. 45. U.S. Department of Health & Human Services; 2009. Code of Federal Regulations, title 45 part 46 subpart A.
-
- McWilliams R, Hoover-Fong J, Hamosh A, Beck S, Beaty T, Cutting G. Problematic variation in local institutional review of a multicenter genetic epidemiology study. JAMA : the journal of the American Medical Association. 2003;290:360–6. - PubMed
-
- Stair TO, Reed CR, Radeos MS, Koski G, Camargo CA Collaboration MIMAR. Variation in institutional review board responses to a standard protocol for a multicenter clinical trial. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2001;8:636–41. - PubMed
-
- Larson E, Bratts T, Zwanziger J, Stone P. A survey of IRB process in 68 U.S. hospitals. Journal of nursing scholarship : an official publication of Sigma Theta Tau International Honor Society of Nursing / Sigma Theta Tau. 2004;36:260–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

