A new structural model of Alzheimer's Aβ42 fibrils based on electron paramagnetic resonance data and Rosetta modeling
- PMID: 26827680
- PMCID: PMC4764428
- DOI: 10.1016/j.jsb.2016.01.013
A new structural model of Alzheimer's Aβ42 fibrils based on electron paramagnetic resonance data and Rosetta modeling
Abstract
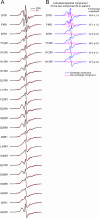
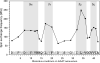
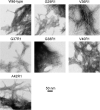
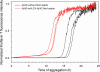
Brain deposition of Aβ in the form of amyloid plaques is a pathological hallmark of Alzheimer's disease. There are two major species of Aβ in the brain: Aβ42 and Aβ40. Although Aβ40 is several-fold more abundant than Aβ42 in soluble form, Aβ42 is the major component of amyloid plaques. Structural knowledge of Aβ42 fibrils is important both for understanding the process of Aβ aggregation and for designing fibril-targeting drugs. Here we report site-specific structural information of Aβ42 fibrils at 22 residue positions based on electron paramagnetic resonance data. In combination with structure prediction program Rosetta, we modeled Aβ42 fibril structure at atomic resolution. Our Aβ42 fibril model consists of four parallel in-register β-sheets: βN (residues ∼7-13), β1 (residues ∼17-20), β2 (residues ∼32-36), and βC (residues 39-41). The region of β1-loop-β2 in Aβ42 fibrils adopts similar structure as that in Aβ40 fibrils. This is consistent with our cross seeding data that Aβ42 fibril seeds shortened the lag phase of Aβ40 fibrillization. On the other hand, Aβ42 fibrils contain a C-terminal β-arc-β motif with a special turn, termed "arc", at residues 37-38, which is absent in Aβ40 fibrils. Our results can explain both the higher aggregation propensity of Aβ42 and the importance of Aβ42 to Aβ40 ratio in the pathogenesis of Alzheimer's disease.
Keywords: Alzheimer disease; Amyloid; Aβ; Protein aggregation; Spin labeling.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Agopian A, Guo Z. Structural origin of polymorphism for Alzheimer's amyloid-β fibrils. Biochem. J. 2012;447:43–50. - PubMed
-
- Antzutkin ON, Leapman RD, Balbach JJ, Tycko R. Supramolecular structural constraints on Alzheimer's β-amyloid fibrils from electron microscopy and solid-state nuclear magnetic resonance. Biochemistry. 2002;41:15436–15450. - PubMed
-
- Baker RT, Catanzariti AM, Karunasekara Y, Soboleva TA, Sharwood R, et al. Using deubiquitylating enzymes as research tools. Methods Enzymol. 2005;398:540–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

