Frequency of dressing changes for central venous access devices on catheter-related infections
- PMID: 26827714
- PMCID: PMC8765739
- DOI: 10.1002/14651858.CD009213.pub2
Frequency of dressing changes for central venous access devices on catheter-related infections
Abstract
Background: People admitted to intensive care units and those with chronic health care problems often require long-term vascular access. Central venous access devices (CVADs) are used for administering intravenous medications and blood sampling. CVADs are covered with a dressing and secured with an adhesive or adhesive tape to protect them from infection and reduce movement. Dressings are changed when they become soiled with blood or start to come away from the skin. Repeated removal and application of dressings can cause damage to the skin. The skin is an important barrier that protects the body against infection. Less frequent dressing changes may reduce skin damage, but it is unclear whether this practice affects the frequency of catheter-related infections.
Objectives: To assess the effect of the frequency of CVAD dressing changes on the incidence of catheter-related infections and other outcomes including pain and skin damage.
Search methods: In June 2015 we searched: The Cochrane Wounds Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In-Process & Other Non-Indexed Citations); Ovid EMBASE and EBSCO CINAHL. We also searched clinical trials registries for registered trials. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria: All randomised controlled trials (RCTs) evaluating the effect of the frequency of CVAD dressing changes on the incidence of catheter-related infections on all patients in any healthcare setting.
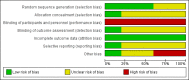
Data collection and analysis: We used standard Cochrane review methodology. Two review authors independently assessed studies for inclusion, performed risk of bias assessment and data extraction. We undertook meta-analysis where appropriate or otherwise synthesised data descriptively when heterogeneous.
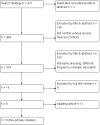
Main results: We included five RCTs (2277 participants) that compared different frequencies of CVAD dressing changes. The studies were all conducted in Europe and published between 1995 and 2009. Participants were recruited from the intensive care and cancer care departments of one children's and four adult hospitals. The studies used a variety of transparent dressings and compared a longer interval between dressing changes (5 to15 days; intervention) with a shorter interval between changes (2 to 5 days; control). In each study participants were followed up until the CVAD was removed or until discharge from ICU or hospital. Confirmed catheter-related bloodstream infection (CRBSI)One trial randomised 995 people receiving central venous catheters to a longer or shorter interval between dressing changes and measured CRBSI. It is unclear whether there is a difference in the risk of CRBSI between people having long or short intervals between dressing changes (RR 1.42, 95% confidence interval (CI) 0.40 to 4.98) (low quality evidence). Suspected catheter-related bloodstream infection Two trials randomised a total of 151 participants to longer or shorter dressing intervals and measured suspected CRBSI. It is unclear whether there is a difference in the risk of suspected CRBSI between people having long or short intervals between dressing changes (RR 0.70, 95% CI 0.23 to 2.10) (low quality evidence). All cause mortalityThree trials randomised a total of 896 participants to longer or shorter dressing intervals and measured all cause mortality. It is unclear whether there is a difference in the risk of death from any cause between people having long or short intervals between dressing changes (RR 1.06, 95% CI 0.90 to 1.25) (low quality evidence). Catheter-site infectionTwo trials randomised a total of 371 participants to longer or shorter dressing intervals and measured catheter-site infection. It is unclear whether there is a difference in risk of catheter-site infection between people having long or short intervals between dressing changes (RR 1.07, 95% CI 0.71 to 1.63) (low quality evidence). Skin damage One small trial (112 children) and three trials (1475 adults) measured skin damage. There was very low quality evidence for the effect of long intervals between dressing changes on skin damage compared with short intervals (children: RR of scoring ≥ 2 on the skin damage scale 0.33, 95% CI 0.16 to 0.68; data for adults not pooled). PainTwo studies involving 193 participants measured pain. It is unclear if there is a difference between long and short interval dressing changes on pain during dressing removal (RR 0.80, 95% CI 0.46 to 1.38) (low quality evidence).
Authors' conclusions: The best available evidence is currently inconclusive regarding whether longer intervals between CVAD dressing changes are associated with more or less catheter-related infection, mortality or pain than shorter intervals.
Conflict of interest statement
Nicole C Gavin: none known Joan Webster: none known Raymond J Chan: none known Claire M Rickard: Claire Rickard is a Board Member of the Australian Intensive Care Foundation. She has carried out consultancy research on IV flushing for Becton Dickinson Medical and has received grants from commercial companies supporting research projects including those on IV dressings. The granting bodies did not undertake study design, procedures, data analysis or preparation of results for publication. She has received payment from commercial companies for educational lectures based on her research, and educational grants to support her conference attendance.
Figures







Update of
- doi: 10.1002/14651858.CD009213
References
References to studies included in this review
Benhamou 2002 {published data only}
-
- Benhamou E, Fessard E, Com‐Nougue C, Beaussier PS, Nitenberg G, Tancrede C, et al. Less frequent catheter dressing changes decrease local cutaneous toxicity of high‐dose chemotherapy in children, without increasing the rate of catheter‐related infections: results of a randomised trial. Bone Marrow Transplantation 2002;29:653‐8. - PubMed
-
- Fessard E. Frequency of change of dressings two comparative trials with central venous catheters in children, hospitalized for severe chemotherapy and bone marrow transplantation [Fréquence de réfection des pansements]. Soins 1994;585/586:33‐8. - PubMed
Engervall 1995 {published data only}
-
- Engervall P, Ringertz S, Hagman E, Skogman K, Bjorkholm M. Change of central venous catheter dressings twice a week is superior to once a week in patients with haematological malignancies. Journal of Hospital Infection 1995;29:275‐86. - PubMed
-
- Engervall P, Ringertz S, Lothman P, Hagman E, Skogman C, Bjorkholm M. A prospective, randomized study comparing change of central venous catheter dressings once or twice a week in patients with hematological malignancies. Journal of Haematology 1994;87(S1):67.
Rasero 2000 {published data only}
-
- Rasero L, Degl'Innocenti M, Mocali M, Alberani F, Boschi S, Giraudi A, et al. Comparison of two different CVC medications change time intervals in bone marrow transplant patients: the results of a multicentre randomised controlled trial [Confronto di due differenti protocolli nell'intervallo del cambio di medicazione per cateteri venosi centrali in pazienti trapiantati di midollo osseo: risultati di uno studio melticentrico randomizzato]. Assistenza Infermieristica e Ricerca 2000;19:122‐9. - PubMed
-
- Rasero L, Degl'Innocenti M, Mocali M, Alberani F, Boschi S, Giraudi A, et al. Comparison of two different time interval protocols for central venous catheter dressing in bone marrow transplant patients: results of a randomized, multicenter study. Haematologica 2000;85:275‐9. - PubMed
Timsit 2009 {published data only}
-
- Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste‐Orgeas M, Pease S, et al. Chlorhexidine‐impregnated sponges and less frequent dressing changes for prevention of catheter‐related infections in critically ill adults. JAMA 2009;301(12):1231‐41. - PubMed
Vokurka 2009 {published data only}
-
- Vokurka S, Bystricka E, Visokaiova M, Scudlova J. Once‐versus twice‐weekly changing of central venous catheter occlusive dressing in intensive chemotherapy patients: results of a randomized multicenter study. Medical Science Monitor 2009;15(3):CR107‐10. - PubMed
References to studies excluded from this review
Bystricka 2004 {published data only}
-
- Bystricka E, Chvojkova I, Suvova J, Mullerova N, Vokurka S, Koza V, et al. Risks and benefit of double‐prolonged interval of central venous catheter dressing changes in patients treated by intensive chemotherapy ‐ proposal of a new randomised nurses' trial. Bone Marrow Transplantation 2004;33(S1):S295.
Davidson 1986 {published data only}
-
- Davidson LE. Dressing subclavian catheters. Nursing Times 1986;82(7):40. - PubMed
Dickerson 1989 {published data only}
-
- Dickerson N, Horton P, Smith S, Rose RC. Clinically significant central venous catheter infections in a community hospital: association with type of dressing. The Journal of Infectious Diseases 1989;160(4):720‐2. - PubMed
Hagerstrom 1994 {published data only}
-
- Hagerstrom M, Matthleson K, Thuessen C. A randomised dressing method for haemodialysis patients with permanent central venous catheters. 4th European Conference on Advances in Wound Management proceedings 1994:abstract 197.
Ishizuka 2011 {published data only}
-
- Ishizuka M, Nagata H, Takagi K, Kubota K. Dressing change reduces the central venous catheter‐related bloodstream infection. Hepato‐Gastroenterology 2011;58:1882‐6. - PubMed
Lucas 1996 {published data only}
-
- Lucas H, Attard‐Montalto S. Central line dressings: study of infection rates. Paediatric Nursing 1996;8(6):21‐3. - PubMed
Powell 1985 {published data only}
-
- Powell CR, Traetow MJ, Fabri PJ, Kudsk KA, Ruberg RL. Op‐site dressing study: a prospective randomized study evaluating povidone iodine ointment and extension set changes with 7‐day op‐site dressings applied to total parenteral nutrition subclavian sites. Journal of Parenteral and Enteral Nutrition 1985;9(3):443‐6. - PubMed
Samsoondar 1985 {published data only}
-
- Samsoondar W, Freeman JB. Colonization of intravascular monitoring devices. Critical Care Medicine 1985;13(9):753‐5. - PubMed
Young 1988 {published data only}
-
- Young GP, Alexeyeff M, Rusell DM, Thomas RJS. Catheter sepsis during parenteral nutrition: the safety of long‐term op‐site dressings. Journal of Parenteral and Enteral Nutrition 1988;12(4):365‐70. - PubMed
Zitella 2003 {published data only}
-
- Zitella L. Central venous catheter site care for blood and marrow transplant recipients. Clinical Journal of Oncology Nursing 2003;7(3):289‐98. - PubMed
References to studies awaiting assessment
Fessard 1994 {published data only}
-
- Fessard E, Baussier PS, Benrondhane S. Prospective randomized trial to study the best time interval between catheter dressing: Study performed by the nurses of pediatric transplantation unit. European Bone Marrow Transplant Nursing Journal 1994;2:7‐9.
Additional references
Camp‐Sorrell 2004
-
- Camp‐Sorrell D. Access Device Guidelines: Recommendations for Nursing Practice and Education. 2nd Edition. Pittsburgh: Oncology Nursing Society, 2004.
Casey 2010
-
- Casey AL, Elliott TSJ. Prevention of central venous catheter‐related infection: update. British Journal of Nursing 2010;19(2):78‐87. - PubMed
CDC 2002
-
- Centers for Disease Control and Prevention. Guidelines for the prevention of intravascular catheter‐related infections. Morbidity and Mortality Weekly Report 2002;51(No. RR‐10):1‐29. - PubMed
CNSA 2007
-
- Cancer Nurses Society of Australia. Central Venous Access Devices: Principles for Nursing Practice and Education. Summary and Recommendations. Cancer Nurses Society of Australia, 2007.
Cutting 2008
-
- Cutting KF. Impact of adhesive surgical tape and wound dressings on the skin, with reference to skin stripping. Journal of Wound Care 2008;17(4):157‐62. - PubMed
DeSpain 1992
-
- DeSpain JD. Dermatologic toxicity of chemotherapy. Seminars in Oncology 1992;19(5):501‐7. - PubMed
Dykes 2001
-
- Dykes PJ, Heggie R, Hill SA. Effects of adhesive dressings on the stratum corneum of skin. Journal of Wound Care 2001;10(2):7‐10. - PubMed
Dykes 2007
-
- Dykes PJ. The effect of adhesive dressing edges on cutaneous irritancy and skin barrier function. Journal of Wound Care 2007;16(3):97‐100. - PubMed
ECOG 2007
-
- Eastern Cooperative Oncology Group. Common Toxicity Criteria. http://ecog.dfci.harvard.edu/general/ctc.pdf (accessed on 16 November 2010).
Elkabir 2001
-
- Elkabir JJ, Khadra A. Wound dehiscence. MRCS Core Modules: Essential Revision Notes. Knutsford: Pastest, 2001.
Elliott 1998
-
- Elliott TS, Tebbs SE. Prevention of central venous catheter related infection. Journal of Hospital Infection 1998;40(3):193‐201. - PubMed
Gabriel 2005
-
- Gabriel J, Bravery K, Doughtery L, Kayley J, Malster M, Scales K. Vascular access: indications and implications for patient care. Nursing Standard 2005;9(26):45‐52. - PubMed
Gillies 2003
Glean 2001
-
- Glean E, Edwards S, Faithfull S, Meredith C, Richards C, Smith M, et al. Interventions for acute radiotherapy induced skin reactions in cancer patients: the development of a clinical guideline recommended for use by the College of Radiographers. Journal of Radiotherapy in Practice 2001;2:75‐84.
Hadaway 2003
-
- Hadaway LC. Infusing without infecting. Nursing 2003;33(10):58‐64. - PubMed
Hayden 2005
-
- Hayden K, Goodman M. Chemotherapy: principles of administration. In: Yarbro CH, Frogge MR, Goodman M editor(s). Cancer Nursing: Principles and Practice. 6th Edition. Boston: Jones and Bartlett, 2005.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Hollingworth 2009
-
- Hollingworth H. Challenges in protecting peri‐wound skin. Nursing Standard 2009;24(7):53‐62. - PubMed
Hopewell 1990
-
- Hopewell JW. The skin: its structure and response to ionizing radiation. International Journal of Radiation Biology 1990;57(4):751‐73. - PubMed
Hunt 1997
-
- Hunt TK, Williams H. Wound healing and wound infection: what surgeons and anesthesiologists can do. Surgical Clinical of North America 1997;77(3):587‐606. - PubMed
INS 2011
-
- Infusion Nursing Society. Infusion nursing standards of practice. Journal of Infusion Nursing 2011;34(S1):S1‐S110.
IVNNZ 2012
-
- Intravenous Nursing New Zealand Incorporated Society. Provisional infusion therapy standards of practice. www.ivnnz.co.nz (accessed 6 August 2015).
Karwoski 2004
-
- Karwoski AC, Plaut RH. Experiments on peeling adhesive tapes from human forearms. Skin Research and Technology 2004;10(4):271‐7. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lotti 1998
-
- Lotti T, Rodofili C, Benci M, Menchin G. Wound healing problems associated with cancers. Journal of Wound Care 1998;7(2):81‐4. - PubMed
Loveday 2014
Maki 1997
-
- Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter‐related bloodstream infection by use of an antiseptic‐impregnated catheter: a randomized, controlled trial. Annals of Internal Medicine 1997;127(4):257‐66. - PubMed
Mermel 2000
-
- Mermel LA. Prevention of intravascular catheter‐related infections. Annals of Internal Medicine 2000;132(5):391‐402. - PubMed
Mermel 2009
O'Grady 2011
-
- O'Grady NP, Alexander M, Burns LA, Patchen Dellinger E, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter‐related infections. http://www.cdc.gov/hicpac/bsi/bsi‐guidelines‐2011.html (accessed 6 August 2015).
O'Rourke 1989
-
- O'Rourke K, Detsky AS. Meta‐analysis in medical research: strong encouragement for higher quality individual research efforts. Journal of Clinical Epidemiology 1989;42(10):1021‐4. - PubMed
Reeves 2011
-
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Rippon 2007
-
- Rippon M, White R, Davies P. Skin adhesives and their role in wound dressings. Wounds UK 2007;3(4):76‐86.
RNAO 2005
-
- Registered Nurses’ Association of Ontario. Care and Maintenance to Reduce Vascular Access Complications. Toronto: Registered Nurses’ Association of Ontario, 2005.
Rosenthal 2003
-
- Rosenthal K. Pinpointing intravascular device infections. Nursing Management 2003;34(6):35‐43. - PubMed
Safdar 2002
-
- Safdar N, Maki DG. Inflammation at the insertion site is not predictive of catheter‐related bloodstream infection with short‐term, noncuffed central venous catheters. Critical Care Medicine 2002;30:2632–5. - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. In: Higgins JPT, Green S (editors). Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
SIGN 2011
-
- Scottish Intercollegiate Guidelines Network. Search filters. http://www.sign.ac.uk/methodology/filters.html (accessed 24 July 2014).
Timsit 2012
-
- Timsit JF, Bouadma L, Ruckly S, Schwebel C, Garrouste‐Orgeas M, Bronchard R, et al. Dressing disruption is a major risk factor for catheter‐related infections. Critical Care Medicine 2012;40:1707‐14. - PubMed
Tortura 2000
-
- Tortura GJ, Grabowski SR. Principles of Anatomy and Physiology. 9th Edition. New York: John Wiley & Sons Inc, 2000.
Walshe 2002
-
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. Journal of Clinical Oncology 2002;20:3276–81. - PubMed
Wille 1993
-
- Wille JC, Blusse van Oud Alblas A, Thewessen EAPM. A comparison of two transparent film‐type dressings on central venous therapy. Journal of Hospital Infection 1993;23(2):113‐21. - PubMed
Wilson 2006
-
- Wilson J. Preventing infection associated with intravascular therapy. Infection Control in Clinical Practice. 3rd Edition. London: Elsevier, 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

