An Update on the Synthesis of Pyrrolo[1,4]benzodiazepines
- PMID: 26828475
- PMCID: PMC6273195
- DOI: 10.3390/molecules21020154
An Update on the Synthesis of Pyrrolo[1,4]benzodiazepines
Abstract
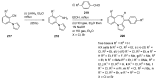
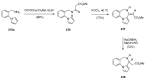
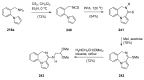
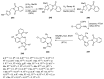
Pyrrolo[1,4]benzodiazepines are tricyclic compounds that are considered "privileged structures" since they possess a wide range of biological activities. The first encounter with these molecules was the isolation of anthramycin from cultures of Streptomyces, followed by determination of the X-ray crystal structure of the molecule and a study of its interaction with DNA. This opened up an intensive synthetic and biological study of the pyrrolo[2,1-c][1,4]benzodiazepines that has culminated in the development of the dimer SJG-136, at present in Phase II clinical trials. The synthetic efforts have brought to light some new synthetic methodology, while the contemporary work is focused on building trimeric pyrrolo[2,1-c][1,4]benzodiazepines linked together by various heterocyclic and aliphatic chains. It is the broad spectrum of biological activities of pyrrolo[1,2-a][1,4]benzodiazepines that has maintained the interest of researchers to date whereas several derivatives of the even less studied pyrrolo[1,2-d][1,4]benzodiazepines were found to be potent non-nucleoside HIV-1 reverse transcriptase inhibitors. The present review is an update on the synthesis of pyrrolo[2,1-c][1,4]benzodiazepines since the last major review of 2011, while the overview of the synthesis of the other two tricyclic isomers is comprehensive.
Keywords: DNA-interactive agents; anti-HIV activity; anticancer activity; heterocycles; pyrrolobenzodiazepines; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




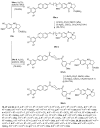




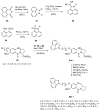
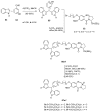
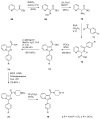


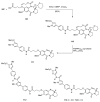



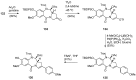
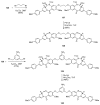


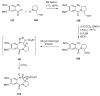
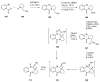

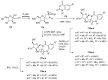


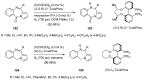
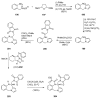














Similar articles
-
Recent developments in novel pyrrolo[2,1-c][1,4]benzodiazepine conjugates: synthesis and biological evaluation.Mini Rev Med Chem. 2003 Jun;3(4):323-39. doi: 10.2174/1389557033488097. Mini Rev Med Chem. 2003. PMID: 12678826 Review.
-
[Research on agents with antineoplastic activity. LXIII. Anthramycin and analogous compounds. X. Synthesis of 11-phenyl-5H-pyrrolo[2,1-c] [1,4] benzodiazepine derivatives].Farmaco Sci. 1979 Oct;34(10):914-22. Farmaco Sci. 1979. PMID: 510534 Italian.
-
Synthesis of C8-linked pyrrolo[2,1-c][1,4]benzodiazepine-benzimidazole conjugates with remarkable DNA-binding affinity.Bioorg Med Chem Lett. 2004 Sep 20;14(18):4791-4. doi: 10.1016/j.bmcl.2004.06.069. Bioorg Med Chem Lett. 2004. PMID: 15324909
-
Synthesis and biological evaluation of an N10-Psec substituted pyrrolo[2,1-c][1,4]benzodiazepine prodrug.Bioorg Med Chem Lett. 2002 May 20;12(10):1413-6. doi: 10.1016/s0960-894x(02)00150-6. Bioorg Med Chem Lett. 2002. PMID: 11992788
-
Synthesis of DNA-interactive pyrrolo[2,1-c][1,4]benzodiazepines (PBDs).Chem Rev. 2011 Apr 13;111(4):2815-64. doi: 10.1021/cr100120f. Epub 2010 Dec 17. Chem Rev. 2011. PMID: 21166464 Review. No abstract available.
Cited by
-
Marine Streptomyces-Derived Novel Alkaloids Discovered in the Past Decade.Mar Drugs. 2024 Jan 22;22(1):51. doi: 10.3390/md22010051. Mar Drugs. 2024. PMID: 38276653 Free PMC article. Review.
-
From Anthramycin to Pyrrolobenzodiazepine (PBD)-Containing Antibody-Drug Conjugates (ADCs).Angew Chem Int Ed Engl. 2017 Jan 9;56(2):462-488. doi: 10.1002/anie.201510610. Epub 2016 Nov 15. Angew Chem Int Ed Engl. 2017. PMID: 27862776 Free PMC article. Review.
-
Immunoconjugates as an Efficient Platform for Drug Delivery: A Resurgence of Natural Products in Targeted Antitumor Therapy.Pharmaceuticals (Basel). 2024 Dec 17;17(12):1701. doi: 10.3390/ph17121701. Pharmaceuticals (Basel). 2024. PMID: 39770542 Free PMC article. Review.
References
-
- Cheeseman G.W.H., Rafiq M. Further cyclisation reactions of 1-arylpyrroles. J. Chem. Soc. C. 1971 doi: 10.1039/j39710002732. - DOI
-
- Yamawaki Y., Watanabe M., Yamamura S., Saito S. Studies on synthetic drugs. II. Syntheses of pyrrolo[1,2-d][1,4]benzodiazepine derivatives. Yakugaku Zasshi. 1977;97:135–142. - PubMed
-
- Thurston D.E. Advances in the study of pyrrolo[2,1-c][1,4]benzodiazepine (PBD) antitumour antibiotics. In: Neidle S., Waring M.J., editors. Molecular Aspects of Anticancer Drug-DNA Interactions. The Macmillan Press Ltd.; London, UK: 1993. pp. 54–88.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

