The Autophagic Machinery in Enterovirus Infection
- PMID: 26828514
- PMCID: PMC4776187
- DOI: 10.3390/v8020032
The Autophagic Machinery in Enterovirus Infection
Abstract
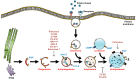
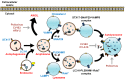
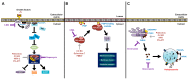
The Enterovirus genus of the Picornaviridae family comprises many important human pathogens, including polioviruses, rhinovirus, enterovirus A71, and enterovirus D68. They cause a wide variety of diseases, ranging from mild to severe life-threatening diseases. Currently, no effective vaccine is available against enteroviruses except for poliovirus. Enteroviruses subvert the autophagic machinery to benefit their assembly, maturation, and exit from host. Some enteroviruses spread between cells via a process described as autophagosome-mediated exit without lysis (AWOL). The early and late phases of autophagy are regulated through various lipids and their metabolizing enzymes. Some of these lipids and enzymes are specifically regulated by enteroviruses. In the present review, we summarize the current understanding of the regulation of autophagic machinery by enteroviruses, and provide updates on recent developments in this field.
Keywords: antiviral; autophagosome maturation; autophagy; enterovirus; lipids; picornavirus; replication.
Figures
Similar articles
-
The life cycle of non-polio enteroviruses and how to target it.Nat Rev Microbiol. 2018 Jun;16(6):368-381. doi: 10.1038/s41579-018-0005-4. Nat Rev Microbiol. 2018. PMID: 29626210 Review.
-
Innate Immunity Evasion by Enteroviruses: Insights into Virus-Host Interaction.Viruses. 2016 Jan 15;8(1):22. doi: 10.3390/v8010022. Viruses. 2016. PMID: 26784219 Free PMC article. Review.
-
Return of the Neurotropic Enteroviruses: Co-Opting Cellular Pathways for Infection.Viruses. 2021 Jan 22;13(2):166. doi: 10.3390/v13020166. Viruses. 2021. PMID: 33499355 Free PMC article. Review.
-
Enterovirus infections with special reference to enterovirus 71.J Microbiol Immunol Infect. 2000 Mar;33(1):1-8. J Microbiol Immunol Infect. 2000. PMID: 10806956 Review.
-
Enterovirus Transmission by Secretory Autophagy.Viruses. 2018 Mar 20;10(3):139. doi: 10.3390/v10030139. Viruses. 2018. PMID: 29558400 Free PMC article. Review.
Cited by
-
MERS-CoV ORF4b is a virulence factor involved in the inflammatory pathology induced in the lungs of mice.PLoS Pathog. 2022 Sep 21;18(9):e1010834. doi: 10.1371/journal.ppat.1010834. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36129908 Free PMC article.
-
The PI3K/Akt/mTOR pathway is involved in CVB3-induced autophagy of HeLa cells.Int J Mol Med. 2017 Jul;40(1):182-192. doi: 10.3892/ijmm.2017.3008. Epub 2017 May 31. Int J Mol Med. 2017. PMID: 28560385 Free PMC article.
-
The interplay of autophagy and enterovirus.Semin Cell Dev Biol. 2020 May;101:12-19. doi: 10.1016/j.semcdb.2019.08.001. Epub 2019 Sep 25. Semin Cell Dev Biol. 2020. PMID: 31563390 Free PMC article. Review.
-
Detection of Viral -RNA and +RNA Strands in Enterovirus-Infected Cells and Tissues.Microorganisms. 2020 Dec 4;8(12):1928. doi: 10.3390/microorganisms8121928. Microorganisms. 2020. PMID: 33291747 Free PMC article.
-
Recent advances on the role of host factors during non-poliovirus enteroviral infections.J Biomed Sci. 2019 Jun 19;26(1):47. doi: 10.1186/s12929-019-0540-y. J Biomed Sci. 2019. PMID: 31215493 Free PMC article. Review.
References
-
- The Picornavirus Pages—Enterovirus. [(accessed on 2 December 2015)]. Available online: http://www.picornaviridae.com/enterovirus/enterovirus.htm.
-
- Symptoms—Non-Polio Enterovirus. [(accessed on 3 December 2015)]; Available online: http://www.cdc.gov/non-polio-enterovirus/about/symptoms.html.
-
- Mallia P., Message S.D., Gielen V., Contoli M., Gray K., Kebadze T., Aniscenko J., Laza-Stanca V., Edwards M.R., Slater L., et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am. J. Crit. Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

