Neuropathogenicity of Two Saffold Virus Type 3 Isolates in Mouse Models
- PMID: 26828718
- PMCID: PMC4734772
- DOI: 10.1371/journal.pone.0148184
Neuropathogenicity of Two Saffold Virus Type 3 Isolates in Mouse Models
Abstract
Objective: Saffold virus (SAFV), a picornavirus, is occasionally detected in children with acute flaccid paralysis, meningitis, and cerebellitis; however, the neuropathogenicity of SAFV remains undetermined.
Methods: The virulence of two clinical isolates of SAFV type 3 (SAFV-3) obtained from a patient with aseptic meningitis (AM strain) and acute upper respiratory inflammation (UR strain) was analyzed in neonatal and young mice utilizing virological, pathological, and immunological methods.
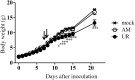
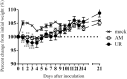
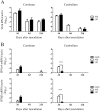
Results: The polyproteins of the strains differed in eight amino acids. Both clinical isolates were infective, exhibited neurotropism, and were mildly neurovirulent in neonatal ddY mice. Both strains pathologically infected neural progenitor cells and glial cells, but not large neurons, with the UR strain also infecting epithelial cells. UR infection resulted in longer inflammation in the brain and spinal cord because of demyelination, while the AM strain showed more infectivity in the cerebellum in neonatal ddY mice. Additionally, young BALB/c mice seroconverted following mucosal inoculation with the UR, but not the AM, strain.
Conclusions: Both SAFV-3 isolates had neurotropism and mild neurovirulence but showed different cell tropisms in both neonatal and young mouse models. This animal model has the potential to recapitulate the potential neuropathogenicity of SAFV-3.
Conflict of interest statement
Figures







Similar articles
-
Intracerebral Inoculation of Mouse-Passaged Saffold Virus Type 3 Affects Cerebellar Development in Neonatal Mice.J Virol. 2016 Oct 14;90(21):10007-10021. doi: 10.1128/JVI.00864-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27581974 Free PMC article.
-
Saffold virus, an emerging human cardiovirus.Rev Med Virol. 2017 Jan;27(1):e1908. doi: 10.1002/rmv.1908. Epub 2016 Oct 10. Rev Med Virol. 2017. PMID: 27723176 Free PMC article. Review.
-
Molecular epidemiological analysis of Saffold cardiovirus genotype 3 from upper respiratory infection patients in Taiwan.J Clin Virol. 2015 Sep;70:7-13. doi: 10.1016/j.jcv.2015.06.100. Epub 2015 Jun 27. J Clin Virol. 2015. PMID: 26305811
-
The Pathogenesis of Saffold Virus in AG129 Mice and the Effects of Its Truncated L Protein in the Central Nervous System.Viruses. 2016 Feb 18;8(2):24. doi: 10.3390/v8020024. Viruses. 2016. PMID: 26901216 Free PMC article.
-
Saffold virus, a novel human Cardiovirus with unknown pathogenicity.J Virol. 2012 Feb;86(3):1292-6. doi: 10.1128/JVI.06087-11. Epub 2011 Nov 23. J Virol. 2012. PMID: 22114344 Free PMC article. Review.
Cited by
-
Intracerebral Inoculation of Mouse-Passaged Saffold Virus Type 3 Affects Cerebellar Development in Neonatal Mice.J Virol. 2016 Oct 14;90(21):10007-10021. doi: 10.1128/JVI.00864-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27581974 Free PMC article.
-
Sequence-specific nanoparticle barcode strategy for multiplex human enterovirus typing.Nat Commun. 2024 Aug 1;15(1):6478. doi: 10.1038/s41467-024-50921-w. Nat Commun. 2024. PMID: 39090126 Free PMC article.
-
Virulence, pathology, and pathogenesis of Pteropine orthoreovirus (PRV) in BALB/c mice: Development of an animal infection model for PRV.PLoS Negl Trop Dis. 2017 Dec 14;11(12):e0006076. doi: 10.1371/journal.pntd.0006076. eCollection 2017 Dec. PLoS Negl Trop Dis. 2017. PMID: 29240753 Free PMC article.
-
Immunohistochemical insights into Saffold virus infection of the brain of juvenile AG129 mice.Virol J. 2016 Nov 25;13(1):191. doi: 10.1186/s12985-016-0654-8. Virol J. 2016. PMID: 27887630 Free PMC article.
-
Saffold virus, an emerging human cardiovirus.Rev Med Virol. 2017 Jan;27(1):e1908. doi: 10.1002/rmv.1908. Epub 2016 Oct 10. Rev Med Virol. 2017. PMID: 27723176 Free PMC article. Review.
References
-
- Craighead JE, McLane MF. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968;162(3856):913–4. Epub 1968/11/22. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

