Automated Protein Localization of Blood Brain Barrier Vasculature in Brightfield IHC Images
- PMID: 26828723
- PMCID: PMC4734698
- DOI: 10.1371/journal.pone.0148411
Automated Protein Localization of Blood Brain Barrier Vasculature in Brightfield IHC Images
Abstract
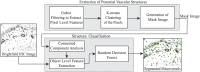
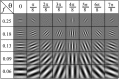
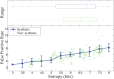
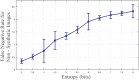
In this paper, we present an objective method for localization of proteins in blood brain barrier (BBB) vasculature using standard immunohistochemistry (IHC) techniques and bright-field microscopy. Images from the hippocampal region at the BBB are acquired using bright-field microscopy and subjected to our segmentation pipeline which is designed to automatically identify and segment microvessels containing the protein glucose transporter 1 (GLUT1). Gabor filtering and k-means clustering are employed to isolate potential vascular structures within cryosectioned slabs of the hippocampus, which are subsequently subjected to feature extraction followed by classification via decision forest. The false positive rate (FPR) of microvessel classification is characterized using synthetic and non-synthetic IHC image data for image entropies ranging between 3 and 8 bits. The average FPR for synthetic and non-synthetic IHC image data was found to be 5.48% and 5.04%, respectively.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

