Evaluating the Impact of Breastfeeding on Rotavirus Antigenemia and Disease Severity in Indian Children
- PMID: 26828823
- PMCID: PMC4734603
- DOI: 10.1371/journal.pone.0146243
Evaluating the Impact of Breastfeeding on Rotavirus Antigenemia and Disease Severity in Indian Children
Abstract
Objectives: To evaluate the contribution of breastfeeding to Rotavirus (RV)-induced antigenemia and/or RNAemia and disease severity in Indian children (<2 yrs age).
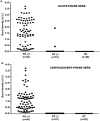
Methods: Paired stool and serum samples were collected from (a) hospitalized infants with diarrhea (n = 145) and (b) healthy control infants without diarrhea (n = 28). Stool RV-antigen was screened in both groups by commercial rapid-test and enzyme immunoassay. The disease severity was scored and real-time-PCR was used for viral-load estimation. Serum was evaluated for RV-antigenemia by EIA and RV-RNAemia by RT-PCR. Data was stratified by age-group and breastfeeding status and compared.
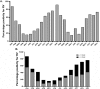
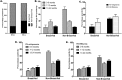
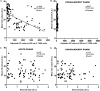
Results: Presence of RV-antigenemia and RV-RNAemia was positively related with presence of RV in stool. Disease severity and stool viral-load was significantly associated with RV-antigenemia [(r = 0.74; CI:0.66 to 0.84; P<0.0001,R2 = 0.59) and (r = -0.55; CI:-0.68 to -0.39; P<0.0001,R2 = 0.31) respectively], but not with RV-RNAemia. There was significant reduction in RV-antigenemiarate in the breast-fed group compared to non-breastfed infants, especially in 0-6 month age group (P<0.001). Non-breastfed infants were at risk for RV-antigenemia with severe disease manifestations in form of high Vesikari scores correlating with high fever, more vomiting episodes and dehydration.
Conclusion: RV-antigenemia was common in nonbreastfed children with severe RV-diarrhea and correlated with stool RV-load and disease severity.
Conflict of interest statement
Figures

Similar articles
-
Rotavirus antigenemia in children is associated with more severe clinical manifestations of acute gastroenteritis.Pediatr Infect Dis J. 2014 Apr;33(4):366-71. doi: 10.1097/INF.0000000000000118. Pediatr Infect Dis J. 2014. PMID: 24136370
-
Rotavirus antigenemia as a common event among children hospitalised for severe, acute gastroenteritis in Belém, northern Brazil.BMC Pediatr. 2019 Jun 12;19(1):193. doi: 10.1186/s12887-019-1535-2. BMC Pediatr. 2019. PMID: 31189470 Free PMC article.
-
Antigenemia, RNAemia, and innate immunity in children with acute rotavirus diarrhea.FEMS Immunol Med Microbiol. 2012 Apr;64(3):382-91. doi: 10.1111/j.1574-695X.2011.00923.x. Epub 2012 Jan 11. FEMS Immunol Med Microbiol. 2012. PMID: 22176605
-
Rotavirus antigenemia in children is associated with viremia.PLoS Med. 2007 Apr;4(4):e121. doi: 10.1371/journal.pmed.0040121. PLoS Med. 2007. PMID: 17439294 Free PMC article.
-
Extraintestinal Involvement of Rotavirus Infection in Children.Arch Iran Med. 2015 Sep;18(9):604-5. Arch Iran Med. 2015. PMID: 26317602 Review.
Cited by
-
Human milk antibodies to global pathogens reveal geographic and interindividual variations in IgA and IgG.J Clin Invest. 2024 Jun 11;134(15):e168789. doi: 10.1172/JCI168789. J Clin Invest. 2024. PMID: 39087469 Free PMC article.
-
Assessment of episodes of pneumonia and diarrhea in vaccinated and unvaccinated children under 60 months of age.Pak J Med Sci. 2020 Nov-Dec;36(7):1596-1600. doi: 10.12669/pjms.36.7.2996. Pak J Med Sci. 2020. PMID: 33235581 Free PMC article.
-
Milk Oligosaccharides Inhibit Human Rotavirus Infectivity in MA104 Cells.J Nutr. 2017 Sep;147(9):1709-1714. doi: 10.3945/jn.116.246090. Epub 2017 Jun 21. J Nutr. 2017. PMID: 28637685 Free PMC article.
-
Rotaviruses: From Pathogenesis to Disease Control-A Critical Review.Viruses. 2022 Apr 22;14(5):875. doi: 10.3390/v14050875. Viruses. 2022. PMID: 35632617 Free PMC article. Review.
-
Relationships among Common Illness Symptoms and the Protective Effect of Breastfeeding in Early Childhood in MAL-ED: An Eight-Country Cohort Study.Am J Trop Med Hyg. 2018 Mar;98(3):904-912. doi: 10.4269/ajtmh.17-0457. Epub 2018 Jan 25. Am J Trop Med Hyg. 2018. PMID: 29380724 Free PMC article.
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. WHO coordinated Global Rotavirus Surveillance Network: 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12(2): 136–41. 10.1016/S1473-3099(11)70253-5 - DOI - PubMed
-
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Costa-Clemens SA, et al., Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354(1):11–22. - PubMed
-
- Blutt SE, Conner ME. Rotavirus: to the gut and beyond! CurrOpinGastroenterol 2007; 23(1): 39–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

