Prevalence of Primary HIV Drug Resistance in Thailand Detected by Short Reverse Transcriptase Genotypic Resistance Assay
- PMID: 26828876
- PMCID: PMC4734770
- DOI: 10.1371/journal.pone.0147945
Prevalence of Primary HIV Drug Resistance in Thailand Detected by Short Reverse Transcriptase Genotypic Resistance Assay
Abstract
Background: HIV drug resistance (HIVDR) is the major cause of treatment failure after scaling up of antiretroviral therapy (ART). HIVDR testing prior to ART initiation is not routinely performed in resource-limited settings. We aimed to assess the prevalence of primary HIVDR by short reverse transcriptase (RT) genotypic resistance assay and evaluate of the impact of the mutations on the treatment outcomes.
Methods: A prospective cohort study was conducted in treatment-naïve HIV-infected patients. Fourteen major mutations of codon 99-191 on the RT gene were selected (K103N, V106A/M, V108I, Q151M, Y181C/I, M184V/I, Y188C/L/H, and G190S/A) at a cost of testing of 35 USD. The association between the presence of primary HIVDR and undetectable HIV RNA (<50 copies/mL) after 6 months of ART was determined.
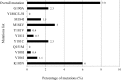
Results: A total of 265 HIV-infected patients were included, with a median age of 35.2 (range, 16.8-75.2) years; 62.6% were males. The median (interquartile range) CD4 cell count at ART initiation was 216 (77-381) cells/mm3. The overall prevalence of primary HIVDR was 7.9%. The prevalence of each HIVDR mutation were K103N 6.0%, V106I 1.1%, V108I 0.4%, Y181C 2.3%, Y181I 0.7%, Y181V 0.4%, M184V 3.0%, M184I 1.5%, and G190A 2.3%. No associated factor of having primary HIVDR was determined. By multiple stepwise logistic regression, factors associated with undetectable HIV RNA after 6 months of ART were: having M184V/I (odds ratio [OR] 0.11; 95% confidence interval [CI] 0.02-0.62, p = 0.013), condom use (OR 2.38; 95% CI 1.12-5.06, p = 0.024), and adherence per 5% increase (OR 1.16; 95% CI 1.00-1.35, p = 0.044).
Conclusions: The prevalence of primary HIVDR is approximately 8%; it is associated with detectable HIV RNA at 6 months after ART initiation. Routine "short RT" genotypic resistance assay should be considered in resource-limited settings to maximize treatment outcome.
Conflict of interest statement
Figures
Similar articles
-
Prevalence and characteristics of HIV drug resistance among antiretroviral treatment (ART) experienced adolescents and young adults living with HIV in Ndola, Zambia.PLoS One. 2020 Aug 17;15(8):e0236156. doi: 10.1371/journal.pone.0236156. eCollection 2020. PLoS One. 2020. PMID: 32804970 Free PMC article.
-
Screening for antiretroviral drug resistance among treatment-naive human immunodeficiency virus type 1-infected individuals in Lebanon.J Infect Dev Ctries. 2014 Mar 13;8(3):339-48. doi: 10.3855/jidc.3593. J Infect Dev Ctries. 2014. PMID: 24619266
-
Comparisons of Primary HIV-1 Drug Resistance between Recent and Chronic HIV-1 Infection within a Sub-Regional Cohort of Asian Patients.PLoS One. 2013 Jun 27;8(6):e62057. doi: 10.1371/journal.pone.0062057. Print 2013. PLoS One. 2013. PMID: 23826076 Free PMC article.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
The utility of integrating nanopore sequencing into routine HIV-1 drug resistance surveillance.Microb Genom. 2025 Mar;11(3):001375. doi: 10.1099/mgen.0.001375. Microb Genom. 2025. PMID: 40111248 Free PMC article. Review.
Cited by
-
Biophysical Characterization of Novel DNA Aptamers against K103N/Y181C Double Mutant HIV-1 Reverse Transcriptase.Molecules. 2022 Jan 3;27(1):285. doi: 10.3390/molecules27010285. Molecules. 2022. PMID: 35011517 Free PMC article.
-
Prevalence of HIV infection, access to HIV care, and response to antiretroviral therapy among partners of HIV-infected individuals in Thailand.PLoS One. 2018 Jun 27;13(6):e0198654. doi: 10.1371/journal.pone.0198654. eCollection 2018. PLoS One. 2018. PMID: 29949594 Free PMC article.
-
HIV Drug Resistance among Pre-treatment Cases in Thailand: Four Rounds of Surveys during 2006-2013.Outbreak Surveill Investig Rep. 2018;11(1):6-13. Outbreak Surveill Investig Rep. 2018. PMID: 30847451 Free PMC article.
-
HIV drug resistance in HIV positive individuals under antiretroviral treatment in Shandong Province, China.PLoS One. 2017 Jul 27;12(7):e0181997. doi: 10.1371/journal.pone.0181997. eCollection 2017. PLoS One. 2017. PMID: 28750025 Free PMC article.
-
Thai national guidelines for the prevention of mother-to-child transmission of human immunodeficiency virus 2017.Asian Biomed (Res Rev News). 2017 Apr;11(2):145-159. doi: 10.5372/1905-7415.1102.547. Asian Biomed (Res Rev News). 2017. PMID: 29861798 Free PMC article.
References
-
- The Joint United Nations Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic 2013. Available: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/....
-
- Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006; 43: 42–46. - PubMed
-
- Jongwutiwes U, Kiertiburanakul S, Sungkanuparph S. Impact of antiretroviral therapy onthe relapse of cryptococcosis and survival of HIV-infected patients with cryptococcal infection. Curr HIV Res. 2007; 5: 355–360. - PubMed
-
- Chasombat S, Lertpiriyasuwat C, Thanprasertsuk S, Suebsaeng L, Lo YR. The National Access to Antiretroviral Program for PHA (NAPHA) in Thailand. Southeast Asian J Trop Med Public Health. 2006; 37: 704–715. - PubMed
-
- Sukasem C, Churdboonchart V, Sirisidthi K, Riengrojpitak S, Chasombat S, Watitpun C, et al. Genotypic resistance mutations in treatment-naïve and treatment-experienced patients under widespread use of antiretroviral drugs in Thailand: implications for further epidemiologic surveillance. Jpn J Infect Dis. 2007; 60: 284–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials