The bacterial dicarboxylate transporter VcINDY uses a two-domain elevator-type mechanism
- PMID: 26828963
- PMCID: PMC5215794
- DOI: 10.1038/nsmb.3166
The bacterial dicarboxylate transporter VcINDY uses a two-domain elevator-type mechanism
Abstract
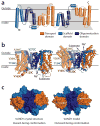
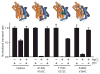
Secondary transporters use alternating-access mechanisms to couple uphill substrate movement to downhill ion flux. Most known transporters use a 'rocking bundle' motion, wherein the protein moves around an immobile substrate-binding site. However, the glutamate-transporter homolog GltPh translocates its substrate-binding site vertically across the membrane, through an 'elevator' mechanism. Here, we used the 'repeat swap' approach to computationally predict the outward-facing state of the Na(+)/succinate transporter VcINDY, from Vibrio cholerae. Our model predicts a substantial elevator-like movement of VcINDY's substrate-binding site, with a vertical translation of ~15 Å and a rotation of ~43°. Our observation that multiple disulfide cross-links completely inhibit transport provides experimental confirmation of the model and demonstrates that such movement is essential. In contrast, cross-links across the VcINDY dimer interface preserve transport, thus revealing an absence of large-scale coupling between protomers.
Figures
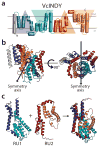


Similar articles
-
Structural basis of ion - substrate coupling in the Na+-dependent dicarboxylate transporter VcINDY.Nat Commun. 2022 May 12;13(1):2644. doi: 10.1038/s41467-022-30406-4. Nat Commun. 2022. PMID: 35551191 Free PMC article.
-
Solvent accessibility changes in a Na+-dependent C4-dicarboxylate transporter suggest differential substrate effects in a multistep mechanism.J Biol Chem. 2020 Dec 25;295(52):18524-18538. doi: 10.1074/jbc.RA120.013894. Epub 2020 Oct 21. J Biol Chem. 2020. PMID: 33087444 Free PMC article.
-
Functional characterization of a Na+-dependent dicarboxylate transporter from Vibrio cholerae.J Gen Physiol. 2014 Jun;143(6):745-59. doi: 10.1085/jgp.201311141. Epub 2014 May 12. J Gen Physiol. 2014. PMID: 24821967 Free PMC article.
-
Shared Molecular Mechanisms of Membrane Transporters.Annu Rev Biochem. 2016 Jun 2;85:543-72. doi: 10.1146/annurev-biochem-060815-014520. Epub 2016 Mar 21. Annu Rev Biochem. 2016. PMID: 27023848 Review.
-
[Molecular characteristics of transporters of C4-dicarboxylates and mechanism of translocation].Zh Evol Biokhim Fiziol. 2009 May-Jun;45(3):263-76. Zh Evol Biokhim Fiziol. 2009. PMID: 19569551 Review. Russian.
Cited by
-
Structural basis of ion - substrate coupling in the Na+-dependent dicarboxylate transporter VcINDY.Nat Commun. 2022 May 12;13(1):2644. doi: 10.1038/s41467-022-30406-4. Nat Commun. 2022. PMID: 35551191 Free PMC article.
-
Elevating the alternating-access model.Nat Struct Mol Biol. 2016 Mar;23(3):187-9. doi: 10.1038/nsmb.3179. Nat Struct Mol Biol. 2016. PMID: 26931415 No abstract available.
-
Structural and biophysical analysis of a Haemophilus influenzae tripartite ATP-independent periplasmic (TRAP) transporter.Elife. 2024 Feb 13;12:RP92307. doi: 10.7554/eLife.92307. Elife. 2024. PMID: 38349818 Free PMC article.
-
Structure and selectivity of a glutamate-specific TAXI TRAP binding protein from Vibrio cholerae.J Gen Physiol. 2024 Dec 2;156(12):e202413584. doi: 10.1085/jgp.202413584. Epub 2024 Nov 18. J Gen Physiol. 2024. PMID: 39556531 Free PMC article.
-
Protein Disulfide Exchange by the Intramembrane Enzymes DsbB, DsbD, and CcdA.J Mol Biol. 2020 Aug 21;432(18):5091-5103. doi: 10.1016/j.jmb.2020.04.008. Epub 2020 Apr 16. J Mol Biol. 2020. PMID: 32305461 Free PMC article. Review.
References
-
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–70. - PubMed
-
- Mitchell P. A general theory of membrane transport from studies of bacteria. Nature. 1957;180:134–6. - PubMed
-
- Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–93. - PubMed
-
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

