Continuous Influx of Genetic Material from Host to Virus Populations
- PMID: 26829124
- PMCID: PMC4735498
- DOI: 10.1371/journal.pgen.1005838
Continuous Influx of Genetic Material from Host to Virus Populations
Abstract
Many genes of large double-stranded DNA viruses have a cellular origin, suggesting that host-to-virus horizontal transfer (HT) of DNA is recurrent. Yet, the frequency of these transfers has never been assessed in viral populations. Here we used ultra-deep DNA sequencing of 21 baculovirus populations extracted from two moth species to show that a large diversity of moth DNA sequences (n = 86) can integrate into viral genomes during the course of a viral infection. The majority of the 86 different moth DNA sequences are transposable elements (TEs, n = 69) belonging to 10 superfamilies of DNA transposons and three superfamilies of retrotransposons. The remaining 17 sequences are moth sequences of unknown nature. In addition to bona fide DNA transposition, we uncover microhomology-mediated recombination as a mechanism explaining integration of moth sequences into viral genomes. Many sequences integrated multiple times at multiple positions along the viral genome. We detected a total of 27,504 insertions of moth sequences in the 21 viral populations and we calculate that on average, 4.8% of viruses harbor at least one moth sequence in these populations. Despite this substantial proportion, no insertion of moth DNA was maintained in any viral population after 10 successive infection cycles. Hence, there is a constant turnover of host DNA inserted into viral genomes each time the virus infects a moth. Finally, we found that at least 21 of the moth TEs integrated into viral genomes underwent repeated horizontal transfers between various insect species, including some lepidopterans susceptible to baculoviruses. Our results identify host DNA influx as a potent source of genetic diversity in viral populations. They also support a role for baculoviruses as vectors of DNA HT between insects, and call for an evaluation of possible gene or TE spread when using viruses as biopesticides or gene delivery vectors.
Conflict of interest statement
The authors have declared that no competing interests exist.
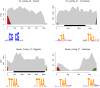
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

