CPEB and miR-15/16 Co-Regulate Translation of Cyclin E1 mRNA during Xenopus Oocyte Maturation
- PMID: 26829217
- PMCID: PMC4734764
- DOI: 10.1371/journal.pone.0146792
CPEB and miR-15/16 Co-Regulate Translation of Cyclin E1 mRNA during Xenopus Oocyte Maturation
Abstract
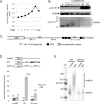
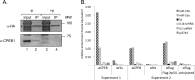
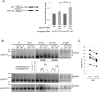
Cell cycle transitions spanning meiotic maturation of the Xenopus oocyte and early embryogenesis are tightly regulated at the level of stored inactive maternal mRNA. We investigated here the translational control of cyclin E1, required for metaphase II arrest of the unfertilised egg and the initiation of S phase in the early embryo. We show that the cyclin E1 mRNA is regulated by both cytoplasmic polyadenylation elements (CPEs) and two miR-15/16 target sites within its 3'UTR. Moreover, we provide evidence that maternal miR-15/16 microRNAs co-immunoprecipitate with CPE-binding protein (CPEB), and that CPEB interacts with the RISC component Ago2. Experiments using competitor RNA and mutated cyclin E1 3'UTRs suggest cooperation of the regulatory elements to sustain repression of the cyclin E1 mRNA during early stages of maturation when CPEB becomes limiting and cytoplasmic polyadenylation of repressed mRNAs begins. Importantly, injection of anti-miR-15/16 LNA results in the early polyadenylation of endogenous cyclin E1 mRNA during meiotic maturation, and an acceleration of GVBD, altogether strongly suggesting that the proximal CPEB and miRNP complexes act to mutually stabilise each other. We conclude that miR-15/16 and CPEB co-regulate cyclin E1 mRNA. This is the first demonstration of the co-operation of these two pathways.
Conflict of interest statement
Figures


References
-
- Kamenska A, Simpson C, Standart N. eIF4E-binding proteins: New factors, new locations, new roles. Bioch Soc Trans. 2014;42:1238–45. - PubMed
-
- Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–85. - PubMed
-
- Minshall N, Reiter M-H, Weil D, Standart N. CPEB interacts with an ovary-specific eIF4E and 4E-T in early Xenopus oocytes. J Biol Chem. 2007;282:37389–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

