β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation
- PMID: 26829388
- PMCID: PMC4767649
- DOI: 10.1038/ncb3307
β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation
Abstract
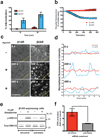
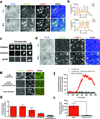
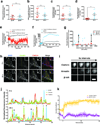
β-Arrestins critically regulate G-protein-coupled receptor (GPCR) signalling, not only 'arresting' the G protein signal but also modulating endocytosis and initiating a discrete G-protein-independent signal through MAP kinase. Despite enormous recent progress towards understanding biophysical aspects of arrestin function, arrestin cell biology remains relatively poorly understood. Two key tenets underlie the prevailing current view: β-arrestin accumulates in clathrin-coated structures (CCSs) exclusively in physical complex with its activating GPCR, and MAP kinase activation requires endocytosis of formed GPCR-β-arrestin complexes. We show here, using β1-adrenergic receptors, that β-arrestin-2 (arrestin 3) accumulates robustly in CCSs after dissociating from its activating GPCR and transduces the MAP kinase signal from CCSs. Moreover, inhibiting subsequent endocytosis of CCSs enhances the clathrin- and β-arrestin-dependent MAP kinase signal. These results demonstrate β-arrestin 'activation at a distance', after dissociating from its activating GPCR, and signalling from CCSs. We propose a β-arrestin signalling cycle that is catalytically activated by the GPCR and energetically coupled to the endocytic machinery.
Figures


Comment in
-
GPCR Signaling: β-arrestins Kiss and Remember.Curr Biol. 2016 Apr 4;26(7):R285-8. doi: 10.1016/j.cub.2016.02.056. Curr Biol. 2016. PMID: 27046816
References
-
- Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28:369–376. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

