Leptin and Pro-Inflammatory Stimuli Synergistically Upregulate MMP-1 and MMP-3 Secretion in Human Gingival Fibroblasts
- PMID: 26829555
- PMCID: PMC4734666
- DOI: 10.1371/journal.pone.0148024
Leptin and Pro-Inflammatory Stimuli Synergistically Upregulate MMP-1 and MMP-3 Secretion in Human Gingival Fibroblasts
Abstract
Introduction: Gingival fibroblast-mediated extracellular matrix remodelling is implicated in the pathogenesis of periodontitis, yet the stimuli that regulate this response are not fully understood. The immunoregulatory adipokine leptin is detectable in the gingiva, human gingival fibroblasts express functional leptin receptor mRNA and leptin is known to regulate extracellular matrix remodelling responses in cardiac fibroblasts. We therefore hypothesised that leptin would enhance matrix metalloproteinase secretion in human gingival fibroblasts.
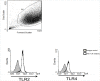
Methods and results: We used in vitro cell culture to investigate leptin signalling and the effect of leptin on mRNA and protein expression in human gingival fibroblasts. We confirmed human gingival fibroblasts expressed cell surface leptin receptor, found leptin increased matrix metalloproteinase-1, -3, -8 and -14 expression in human gingival fibroblasts compared to unstimulated cells, and observed that leptin stimulation activated MAPK, STAT1/3 and Akt signalling in human gingival fibroblasts. Furthermore, leptin synergised with IL-1 or the TLR2 agonist pam2CSK4 to markedly enhance matrix metalloproteinase-1 and -3 production by human gingival fibroblasts. Signalling pathway inhibition demonstrated ERK was required for leptin-stimulated matrix metalloproteinase-1 expression in human gingival fibroblasts; whilst ERK, JNK, p38 and STAT3 were required for leptin+IL-1- and leptin+pam2CSK4-induced matrix metalloproteinase-1 expression. A genome-wide expression array and gene ontology analysis confirmed genes differentially expressed in leptin+IL-1-stimulated human gingival fibroblasts (compared to unstimulated cells) were enriched for extracellular matrix organisation and disassembly, and revealed that matrix metalloproteinase-8 and -12 were also synergistically upregulated by leptin+IL-1 in human gingival fibroblasts.
Conclusions: We conclude that leptin selectively enhances the expression and secretion of certain matrix metalloproteinases in human gingival fibroblasts, and suggest that gingival fibroblasts may have an ECM-degrading phenotype during conditions of hyperleptinaemia (e.g., obesity, type 2 diabetes mellitus, exogenous leptin therapy).
Conflict of interest statement
Figures





Similar articles
-
Enamel matrix derivative induces the expression of tissue inhibitor of matrix metalloproteinase-3 in human gingival fibroblasts via extracellular signal-regulated kinase.J Periodontal Res. 2010 Apr;45(2):200-6. doi: 10.1111/j.1600-0765.2009.01218.x. Epub 2009 Nov 9. J Periodontal Res. 2010. PMID: 19909407
-
Extracellular β-NAD(+) inhibits interleukin-1-induced matrix metalloproteinase-1 and -3 expression on human gingival fibroblasts.Connect Tissue Res. 2013;54(3):204-9. doi: 10.3109/03008207.2013.782013. Epub 2013 Apr 15. Connect Tissue Res. 2013. PMID: 23509928
-
Transforming growth factor-beta induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase.J Biol Chem. 1999 Dec 24;274(52):37292-300. doi: 10.1074/jbc.274.52.37292. J Biol Chem. 1999. PMID: 10601295
-
Phenotypic study of human gingival fibroblasts labeled with superparamagnetic anionic nanoparticles.J Periodontol. 2006 Feb;77(2):238-47. doi: 10.1902/jop.2006.050064. J Periodontol. 2006. PMID: 16460250
-
The phenotype of gingival fibroblasts and their potential use in advanced therapies.Eur J Cell Biol. 2020 Sep;99(7):151123. doi: 10.1016/j.ejcb.2020.151123. Epub 2020 Sep 7. Eur J Cell Biol. 2020. PMID: 33070040 Review.
Cited by
-
Leptin-induced signaling pathways in cancer cell migration and invasion.Cell Oncol (Dordr). 2019 Jun;42(3):243-260. doi: 10.1007/s13402-019-00428-0. Epub 2019 Mar 15. Cell Oncol (Dordr). 2019. PMID: 30877623 Review.
-
The Emerging Role of MMP12 in the Oral Environment.Int J Mol Sci. 2023 Feb 28;24(5):4648. doi: 10.3390/ijms24054648. Int J Mol Sci. 2023. PMID: 36902078 Free PMC article. Review.
-
PGC-1α-mediated mitochondrial homeostasis imbalance aggravated gingival lesions of diabetic periodontitis by disrupting ECM remodeling of human gingival fibroblast.Redox Biol. 2025 Aug 22;86:103829. doi: 10.1016/j.redox.2025.103829. Online ahead of print. Redox Biol. 2025. PMID: 40865270 Free PMC article.
-
The relationship between leptin and periodontitis: a literature review.PeerJ. 2023 Dec 15;11:e16633. doi: 10.7717/peerj.16633. eCollection 2023. PeerJ. 2023. PMID: 38111655 Free PMC article. Review.
-
Differential Matrix Metalloprotease (MMP) Expression Profiles Found in Aged Gingiva.PLoS One. 2016 Jul 8;11(7):e0158777. doi: 10.1371/journal.pone.0158777. eCollection 2016. PLoS One. 2016. PMID: 27391467 Free PMC article. Clinical Trial.
References
-
- Bartold PM, Narayanan AS. (2006) Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol 2000 40: 29–49. - PubMed
-
- Nagase H, Visse R, Murphy G. (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562–573. - PubMed
-
- Bodet C, Andrian E, Tanabe S-i, Grenier D. (2007) Actinobacillus actinomycetemcomitans lipopolysaccharide regulates matrix metalloproteinase, tissue inhibitors of matrix metalloproteinase, and plasminogen activator production by human gingival fibroblasts: A potential role in connective tissue destruction. Journal of Cellular Physiology 212: 189–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

