Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants
- PMID: 26829877
- PMCID: PMC4963074
- DOI: 10.1080/21645515.2015.1136040
Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants
Abstract
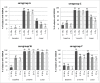
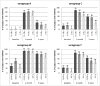
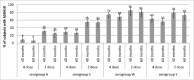
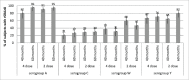
Persistence of bactericidal antibodies following vaccination is extremely important for protection against invasive meningococcal disease, given the epidemiology and rapid progression of meningococcal infection. We present an analysis of antibody persistence and booster response to MenACWY-CRM, in adolescents, children and infants, from 7 clinical studies. Immunogenicity was assessed using the serum bactericidal assay with both human and rabbit complement. Post-vaccination hSBA titers were high, with an age- and serogroup-specific decline in titers up to 1 y and stable levels up to 5 y The waning of hSBA titers over time was more pronounced among infants and toddlers and the greatest for serogroup A. However, rSBA titers against serogroup A were consistently higher and showed little decline over time, suggesting that protection against this serogroup may be sustained. A single booster dose of MenACWY-CRM administered at 3 to 5 y induced a robust immune response in all age groups.
Keywords: MenACWY-CRM; booster; hSBA; meningococcal; persistence; rSBA.
Figures

Similar articles
-
Antibody persistence and booster response following MenACWY-CRM vaccination in children as assessed by two different assay methods.Vaccine. 2019 Jul 26;37(32):4460-4467. doi: 10.1016/j.vaccine.2019.06.076. Epub 2019 Jul 3. Vaccine. 2019. PMID: 31279564 Clinical Trial.
-
Safety and immunogenicity of a booster dose of meningococcal (groups A, C, W, and Y) polysaccharide diphtheria toxoid conjugate vaccine.Vaccine. 2016 Oct 17;34(44):5273-5278. doi: 10.1016/j.vaccine.2016.09.003. Epub 2016 Sep 15. Vaccine. 2016. PMID: 27642132 Clinical Trial.
-
Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents.J Pediatr. 2014 Jun;164(6):1409-15.e4. doi: 10.1016/j.jpeds.2014.02.025. Epub 2014 Mar 20. J Pediatr. 2014. PMID: 24657122 Clinical Trial.
-
MenACWY-TT vaccine for active immunization against invasive meningococcal disease.Expert Rev Vaccines. 2012 May;11(5):523-37. doi: 10.1586/erv.12.32. Expert Rev Vaccines. 2012. PMID: 22827239 Review.
-
An update of clinical experience with the quadrivalent meningococcal ACWY-CRM conjugate vaccine.Expert Rev Vaccines. 2018 Oct;17(10):865-880. doi: 10.1080/14760584.2018.1521280. Epub 2018 Sep 27. Expert Rev Vaccines. 2018. PMID: 30198805 Review.
Cited by
-
A phase 2b/3b MenACWY-TT study of long-term antibody persistence after primary vaccination and immunogenicity and safety of a booster dose in individuals aged 11 through 55 years.BMC Infect Dis. 2020 Jun 18;20(1):426. doi: 10.1186/s12879-020-05104-5. BMC Infect Dis. 2020. PMID: 32552685 Free PMC article. Clinical Trial.
-
One-year antibody persistence and safety of a 4-dose schedule of MenACWY-CRM in healthy infants from South Korea.Clin Exp Vaccine Res. 2019 Jul;8(2):94-102. doi: 10.7774/cevr.2019.8.2.94. Epub 2019 Jul 31. Clin Exp Vaccine Res. 2019. PMID: 31406690 Free PMC article.
-
Progress toward the global control of Neisseria meningitidis: 21st century vaccines, current guidelines, and challenges for future vaccine development.Hum Vaccin Immunother. 2018 May 4;14(5):1146-1160. doi: 10.1080/21645515.2018.1451810. Epub 2018 May 9. Hum Vaccin Immunother. 2018. PMID: 29543582 Free PMC article. Review.
-
Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study.Hum Vaccin Immunother. 2020 Jun 2;16(6):1292-1298. doi: 10.1080/21645515.2020.1733867. Epub 2020 Mar 25. Hum Vaccin Immunother. 2020. PMID: 32209015 Free PMC article. Clinical Trial.
-
Modeling antibody persistence after MenACYW-TT vaccination and comparative analysis with other quadrivalent meningococcal vaccines.Sci Rep. 2025 Jul 10;15(1):24990. doi: 10.1038/s41598-025-08112-0. Sci Rep. 2025. PMID: 40640249 Free PMC article.
References
-
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27 Suppl 2:B51-863; PMID:19477562; http://dx.doi.org/10.1016/j.vaccine.2009.04.063 - DOI - PubMed
-
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853-61; PMID:21075057; http://dx.doi.org/10.1016/S1473-3099(10)70251-6 - DOI - PubMed
-
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention (CDC) . Prevention and control of meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Recommendations Rep March 2013. 66(RR02);1-22 [accessed November2015]. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm - PubMed
-
- National Health Service, UK The NHS vaccination schedule, April 2014. [accessed November2015]. Available at: http://www.nhs.uk/Conditions/vaccinations/Pages/vaccination-schedule-age...
-
- Departamento de Actualización Profesional Boletín Official 33.085, 12 March 2015. [accessed November2015] Available at http://www.colfarsfe.org.ar/newsfiles/marzo2015/Disposiciones12-03-15.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
