Precursor and mature NGF live tracking: one versus many at a time in the axons
- PMID: 26829890
- PMCID: PMC4735336
- DOI: 10.1038/srep20272
Precursor and mature NGF live tracking: one versus many at a time in the axons
Erratum in
-
Corrigendum: Precursor and mature NGF live tracking: one versus many at a time in the axons.Sci Rep. 2016 Mar 30;6:23308. doi: 10.1038/srep23308. Sci Rep. 2016. PMID: 27027850 Free PMC article. No abstract available.
Abstract
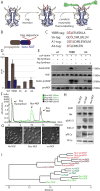
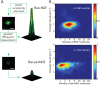
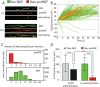
The classical view of nerve growth factor (NGF) action in the nervous system is linked to its retrograde axonal transport. However, almost nothing is known on the trafficking properties of its unprocessed precursor proNGF, characterized by different and generally opposite biological functions with respect to its mature counterpart. Here we developed a strategy to fluorolabel both purified precursor and mature neurotrophins (NTs) with a controlled stoichiometry and insertion site. Using a single particle tracking approach, we characterized the axonal transport of proNGF versus mature NGF in living dorsal root ganglion neurons grown in compartmentalized microfluidic devices. We demonstrate that proNGF is retrogradely transported as NGF, but with a lower flux and a different distribution of numbers of neurotrophins per vesicle. Moreover, exploiting a dual-color labelling technique, we analysed the transport of both NT forms when simultaneously administered to the axon tips.
Figures

References
-
- Nykjaer A., Willnow T. E. & Petersen C. M. p75NTR-live or let die. Curr. Opin. Neurobiol. 15, 49–57 (2005). - PubMed
-
- Cattaneo A. & Calissano P. Nerve growth factor and Alzheimer’s disease: new facts for an old hypothesis. Mol. Neurobiol. 46, 588–604 (2012). - PubMed
-
- Hempstead B. L. Dissecting the diverse actions of pro- and mature neurotrophins. Curr. Alzheimer Res. 3, 19–24 (2006). - PubMed
-
- Iulita M. F. & Cuello A. C. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol. Sci. 35, 338–48 (2014). - PubMed
-
- Fahnestock M., Michalski B., Xu B. & Coughlin M. D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell. Neurosci. 18, 210–220 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

