Neuropsychological, Neurovirological and Neuroimmune Aspects of Abnormal GABAergic Transmission in HIV Infection
- PMID: 26829944
- PMCID: PMC4848342
- DOI: 10.1007/s11481-016-9652-2
Neuropsychological, Neurovirological and Neuroimmune Aspects of Abnormal GABAergic Transmission in HIV Infection
Abstract
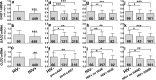
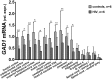
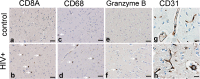
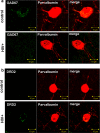
The prevalence of HIV-associated neurocognitive disorders (HAND) remains high in patients with effective suppression of virus replication by combination antiretroviral therapy (cART). Several neurotransmitter systems were reported to be abnormal in HIV-infected patients, including the inhibitory GABAergic system, which mediates fine-tuning of neuronal processing and plays an essential role in cognitive functioning. To elucidate the role of abnormal GABAergic transmission in HAND, the expression of GABAergic markers was measured in 449 human brain specimens from HIV-infected patients with and without HAND. Using real-time polymerase chain reaction, immunoblotting and immunohistochemistry we found that the GABAergic markers were significantly decreased in most sectors of cerebral neocortex, the neostriatum, and the cerebellum of HIV-infected subjects. Low GABAergic expression in frontal neocortex was correlated significantly with high expression of endothelial cell markers, dopamine receptor type 2 (DRD2L), and preproenkephalin (PENK) mRNAs, and with worse performance on tasks of verbal fluency. Significant associations were not found between low GABAergic mRNAs and HIV-1 RNA concentration in the brain, the history of cART, or HIV encephalitis. Pathological evidence of neurodegeneration of the affected GABAergic neurons was not present. We conclude that abnormally low expression of GABAergic markers is prevalent in HIV-1 infected patients. Interrelationships with other neurotransmitter systems including dopaminergic transmission and with endothelial cell markers lend added support to suggestions that synaptic plasticity and cerebrovascular anomalies are involved with HAND in virally suppressed patients.
Keywords: Autopsy; GABA; GABAergic; Glutamate decarboxylase; HIV associated neurocognitive disorders; HIV encephalitis.
Figures


Similar articles
-
Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis.J Neuroimmune Pharmacol. 2012 Sep;7(3):686-700. doi: 10.1007/s11481-012-9345-4. Epub 2012 Mar 6. J Neuroimmune Pharmacol. 2012. PMID: 22391864 Free PMC article.
-
Cannabinoids Occlude the HIV-1 Tat-Induced Decrease in GABAergic Neurotransmission in Prefrontal Cortex Slices.J Neuroimmune Pharmacol. 2016 Jun;11(2):316-31. doi: 10.1007/s11481-016-9664-y. Epub 2016 Mar 18. J Neuroimmune Pharmacol. 2016. PMID: 26993829 Free PMC article.
-
Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort.J Acquir Immune Defic Syndr. 2013 Apr 15;62(5):487-95. doi: 10.1097/QAI.0b013e31827f1bdb. J Acquir Immune Defic Syndr. 2013. PMID: 23242157 Free PMC article.
-
When do models of NeuroAIDS faithfully imitate "the real thing"?J Neurovirol. 2018 Apr;24(2):146-155. doi: 10.1007/s13365-017-0601-5. Epub 2017 Dec 18. J Neurovirol. 2018. PMID: 29256039 Free PMC article. Review.
-
HIV Infection and Neurocognitive Disorders in the Context of Chronic Drug Abuse: Evidence for Divergent Findings Dependent upon Prior Drug History.J Neuroimmune Pharmacol. 2020 Dec;15(4):715-728. doi: 10.1007/s11481-020-09928-5. Epub 2020 Jun 12. J Neuroimmune Pharmacol. 2020. PMID: 32533296 Free PMC article. Review.
Cited by
-
Inhibitory Neurotransmission Is Sex-Dependently Affected by Tat Expression in Transgenic Mice and Suppressed by the Fatty Acid Amide Hydrolase Enzyme Inhibitor PF3845 via Cannabinoid Type-1 Receptor Mechanisms.Cells. 2022 Mar 2;11(5):857. doi: 10.3390/cells11050857. Cells. 2022. PMID: 35269478 Free PMC article.
-
Dopaminergic impact of cART and anti-depressants on HIV neuropathogenesis in older adults.Brain Res. 2019 Nov 15;1723:146398. doi: 10.1016/j.brainres.2019.146398. Epub 2019 Aug 21. Brain Res. 2019. PMID: 31442412 Free PMC article. Review.
-
Chronic HIV-1 Tat exposure alters anterior cingulate cortico-basal ganglia-thalamocortical synaptic circuitry, associated behavioral control, and immune regulation in male mice.Brain Behav Immun Health. 2020 May;5:100077. doi: 10.1016/j.bbih.2020.100077. Epub 2020 Apr 29. Brain Behav Immun Health. 2020. PMID: 33083793 Free PMC article.
-
HIV and opiates dysregulate K+- Cl- cotransporter 2 (KCC2) to cause GABAergic dysfunction in primary human neurons and Tat-transgenic mice.Neurobiol Dis. 2020 Jul;141:104878. doi: 10.1016/j.nbd.2020.104878. Epub 2020 Apr 25. Neurobiol Dis. 2020. PMID: 32344154 Free PMC article.
-
Advances in the Experimental Models of HIV-Associated Neurological Disorders.Curr HIV/AIDS Rep. 2021 Oct;18(5):459-474. doi: 10.1007/s11904-021-00570-1. Epub 2021 Aug 24. Curr HIV/AIDS Rep. 2021. PMID: 34427869 Free PMC article. Review.
References
-
- Ances BM, Vaida F, Cherner M, Yeh MJ, Liang CL, Gardner C, Grant I, Ellis RJ, Buxton RB, HIV Neurobehavioral Research Center (HNRC) Group HIV and chronic methamphetamine dependence affect cerebral blood flow. J Neuroimmune Pharmacol. 2011;6:409–419. doi: 10.1007/s11481-011-9270-y. - DOI - PMC - PubMed
-
- Anneken JH, Cunningham JI, Collins SA, Yamamoto BK, Gudelsky GA. MDMA increases glutamate release and reduces parvalbumin-positive GABAergic cells in the dorsal hippocampus of the rat: role of cyclooxygenase. J Neuroimmune Pharmacol. 2013;8:58–65. doi: 10.1007/s11481-012-9420-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

