Changes in intestinal microflora of Caenorhabditis elegans following Bacillus nematocida B16 infection
- PMID: 26830015
- PMCID: PMC4735852
- DOI: 10.1038/srep20178
Changes in intestinal microflora of Caenorhabditis elegans following Bacillus nematocida B16 infection
Abstract
The effect of pathogenic bacteria on a host and its symbiotic microbiota is vital and widespread in the biotic world. The soil-dwelling opportunistic bacterium Bacillus nematocida B16 uses a "Trojan horse" mechanism to kill Caenorhabditis elegans. The alterations in the intestinal microflora that occur after B16 infection remain unknown. Here, we analyzed the intestinal bacteria presented in normal and infected worms. The gut microbial community experienced a complex change after B16 inoculation, as determined through marked differences in species diversity, structure, distribution and composition between uninfected and infected worms. Regardless of the worm's origin (i.e., from soil or rotten fruits), the diversity of the intestinal microbiome decreased after infection. Firmicutes increased sharply, whereas Proteobacteria, Actinobacteria, Cyanobacteria and Acidobacteria decreased to different degrees. Fusobacteria was only present 12 h post-infection. After 24 h of infection, 1228 and 1109 bacterial species were identified in the uninfected and infected groups, respectively. The shared species reached 21.97%. The infected group had a greater number of Bacillus species but a smaller number of Pediococcus, Halomonas, Escherichia and Shewanella species (P < 0.01). Therefore, this study provides the first evaluation of the alterations caused by pathogenic bacteria on symbiotic microbiota using C. elegans as the model species.
Figures


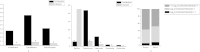


Similar articles
-
Stenotrophomonas strain CPCC 101271, an intestinal lifespan-prolonging bacterium for Caenorhabditis elegans that assists in host resistance to "Bacillus nematocida" colonization.Arch Microbiol. 2021 Oct;203(8):4951-4960. doi: 10.1007/s00203-021-02467-4. Epub 2021 Jul 14. Arch Microbiol. 2021. PMID: 34258643 Free PMC article.
-
Whole-genome analysis of the colonization-resistant bacterium Phytobacter sp. SCO41T isolated from Bacillus nematocida B16-fed adult Caenorhabditis elegans.Mol Biol Rep. 2019 Apr;46(2):1563-1575. doi: 10.1007/s11033-018-04574-w. Epub 2019 Mar 16. Mol Biol Rep. 2019. PMID: 30879274
-
Colonization of Caenorhabditis elegans by Bacillus nematocida B16, a bacterial opportunistic pathogen.J Mol Microbiol Biotechnol. 2012;22(4):258-67. doi: 10.1159/000342911. Epub 2012 Oct 4. J Mol Microbiol Biotechnol. 2012. PMID: 23037141
-
Caenorhabditis elegans as a Convenient Animal Model for Microbiome Studies.Int J Mol Sci. 2024 Jun 18;25(12):6670. doi: 10.3390/ijms25126670. Int J Mol Sci. 2024. PMID: 38928375 Free PMC article. Review.
-
[The nematode Caenorhabditis elegans as a model for the study of host-pathogen interactions].J Soc Biol. 2003;197(4):375-8. J Soc Biol. 2003. PMID: 15005519 Review. French.
Cited by
-
Zearalenone Changes the Diversity and Composition of Caecum Microbiota in Weaned Rabbit.Biomed Res Int. 2018 Oct 8;2018:3623274. doi: 10.1155/2018/3623274. eCollection 2018. Biomed Res Int. 2018. PMID: 30402473 Free PMC article.
-
The bacterial community associated with the sheep gastrointestinal nematode parasite Haemonchus contortus.PLoS One. 2018 Feb 8;13(2):e0192164. doi: 10.1371/journal.pone.0192164. eCollection 2018. PLoS One. 2018. PMID: 29420571 Free PMC article.
-
The Shift of the Intestinal Microbiome in the Innate Immunity-Deficient Mutant rde-1 Strain of C. elegans upon Orsay Virus Infection.Front Microbiol. 2017 May 29;8:933. doi: 10.3389/fmicb.2017.00933. eCollection 2017. Front Microbiol. 2017. PMID: 28611740 Free PMC article.
-
Methods to extract and study the biological effects of murine gut microbiota using Caenorhabditis elegans as a screening host.PLoS One. 2023 Feb 23;18(2):e0281887. doi: 10.1371/journal.pone.0281887. eCollection 2023. PLoS One. 2023. PMID: 36821579 Free PMC article.
-
Phytobacter diazotrophicus from Intestine of Caenorhabditis elegans Confers Colonization-Resistance against Bacillus nematocida Using Flagellin (FliC) as an Inhibition Factor.Pathogens. 2022 Jan 10;11(1):82. doi: 10.3390/pathogens11010082. Pathogens. 2022. PMID: 35056030 Free PMC article.
References
-
- Huang X., Niu Q., Zhou W. & Zhang. K. Bacillus nematocida sp. nov., a novel bacterial strain with nematotoxic activity isolated from soil in Yunnan, China. Syst Appl Microbiol 28, 323–327 (2005). - PubMed
-
- Niu Q. et al.. Bacillus sp. B16 kills nematodes with a serine protease identified as a pathogenic factor. Appl Microbiol Biot. 69, 722–730 (2006a). - PubMed
-
- Niu Q. et al.. A neutral protease from Bacillus nematocida, another potential virulence factor in the infection against nematodes. Arch Microbiol. 185, 439–448 (2006b). - PubMed
-
- Niu Q. et al.. Functional identification of the gene bace16 from nematophagous bacterium Bacillus nematocida. Appl Microbiol Biot. 75, 141–148 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

