Krypton oxides under pressure
- PMID: 26830129
- PMCID: PMC4735652
- DOI: 10.1038/srep18938
Krypton oxides under pressure
Abstract
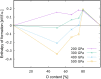
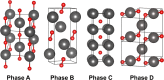
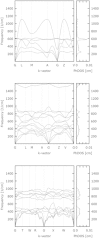
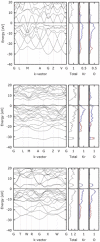
Under high pressure, krypton, one of the most inert elements is predicted to become sufficiently reactive to form a new class of krypton compounds; krypton oxides. Using modern ab-initio evolutionary algorithms in combination with Density Functional Theory, we predict the existence of several thermodynamically stable Kr/O species at elevated pressures. In particular, our calculations indicate that at approx. 300 GPa the monoxide, KrO, should form spontaneously and remain thermo- and dynamically stable with respect to constituent elements and higher oxides. The monoxide is predicted to form non-molecular crystals with short Kr-O contacts, typical for genuine chemical bonds.
Figures

References
-
- Rayleigh L. & Ramsay W. Argon, a New Constituent of the Atmosphere. Proc. R. Soc. 57, 265–287 (1894).
-
- Pauling L. The Formulas of Antimonic Acid and the Antimonates. J. Am. Chem. Soc. 55, 1895–1900 (1933).
-
- Bartlett N. Xenon hexafluoroplatinate (V) Xe+[PtF6]−. Proc. Chem. Soc. 218, 197–236 (1962).
-
- MacKenzie D. R. Krypton Difluoride: Preparation and Handling. Science 141, 1171–1172 (1963). - PubMed
-
- Lundell J. & Kunttu H. Structure, spectra, and stability of argon hydride ion (Ar2H+), krypton hydride ion (Kr2H+), xenon hydride ion (Xe2H+): an effective core potential approach. J. Phys. Chem. 96, 9774–9781 (1992).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

