Arrested Hematopoiesis and Vascular Relaxation Defects in Mice with a Mutation in Dhfr
- PMID: 26830229
- PMCID: PMC4836279
- DOI: 10.1128/MCB.01035-15
Arrested Hematopoiesis and Vascular Relaxation Defects in Mice with a Mutation in Dhfr
Abstract
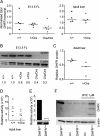
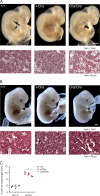
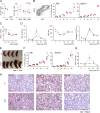
Dihydrofolate reductase (DHFR) is a critical enzyme in the folate metabolism pathway and also plays a role in regulating nitric oxide (NO) signaling in endothelial cells. Although both coding and noncoding mutations with phenotypic effects have been identified in the human DHFR gene, no mouse model is currently available to study the consequences of perturbing DHFR in vivo In order to identify genes involved in definitive hematopoiesis, we performed a forward genetic screen and produced a mouse line, here referred to as Orana, with a point mutation in the Dhfr locus leading to a Thr136Ala substitution in the DHFR protein. Homozygote Orana mice initiate definitive hematopoiesis, but expansion of progenitors in the fetal liver is compromised, and the animals die between embryonic day 13.5 (E13.5) and E14.5. Heterozygote Orana mice survive to adulthood but have tissue-specific alterations in folate abundance and distribution, perturbed stress erythropoiesis, and impaired endothelium-dependent relaxation of the aorta consistent with the role of DHFR in regulating NO signaling. Orana mice provide insight into the dual roles of DHFR and are a useful model for investigating the role of environmental and dietary factors in the context of vascular defects caused by altered NO signaling.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Endothelial Nitric Oxide Synthase-Derived Nitric Oxide Prevents Dihydrofolate Reductase Degradation via Promoting S-Nitrosylation.Arterioscler Thromb Vasc Biol. 2015 Nov;35(11):2366-73. doi: 10.1161/ATVBAHA.115.305796. Epub 2015 Sep 17. Arterioscler Thromb Vasc Biol. 2015. PMID: 26381869 Free PMC article.
-
Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease.Am J Hum Genet. 2011 Feb 11;88(2):226-31. doi: 10.1016/j.ajhg.2011.01.007. Am J Hum Genet. 2011. PMID: 21310277 Free PMC article.
-
Dihydrofolate reductase is required for the development of heart and outflow tract in zebrafish.Acta Biochim Biophys Sin (Shanghai). 2011 Dec;43(12):957-69. doi: 10.1093/abbs/gmr098. Acta Biochim Biophys Sin (Shanghai). 2011. PMID: 22113051
-
Regulation of human dihydrofolate reductase activity and expression.Vitam Horm. 2008;79:267-92. doi: 10.1016/S0083-6729(08)00409-3. Vitam Horm. 2008. PMID: 18804698 Review.
-
Molecular mechanisms of resistance to antifolates, a review.Acta Biochim Pol. 1995;42(4):457-64. Acta Biochim Pol. 1995. PMID: 8852336 Review.
Cited by
-
Knockout of dihydrofolate reductase in mice induces hypertension and abdominal aortic aneurysm via mitochondrial dysfunction.Redox Biol. 2019 Jun;24:101185. doi: 10.1016/j.redox.2019.101185. Epub 2019 Mar 29. Redox Biol. 2019. PMID: 30954686 Free PMC article.
-
Non-alcoholic fatty liver disease, vascular inflammation and insulin resistance are exacerbated by TRAIL deletion in mice.Sci Rep. 2017 May 15;7(1):1898. doi: 10.1038/s41598-017-01721-4. Sci Rep. 2017. PMID: 28507343 Free PMC article.
-
Deciphering the role of miRNA-mRNA interactions in cerebral vasospasm post intracranial hemorrhage.Front Mol Biosci. 2025 Feb 6;12:1492729. doi: 10.3389/fmolb.2025.1492729. eCollection 2025. Front Mol Biosci. 2025. PMID: 39981435 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
