Palisade Endings Are a Constant Feature in the Extraocular Muscles of Frontal-Eyed, But Not Lateral-Eyed, Animals
- PMID: 26830369
- PMCID: PMC4826744
- DOI: 10.1167/iovs.15-18716
Palisade Endings Are a Constant Feature in the Extraocular Muscles of Frontal-Eyed, But Not Lateral-Eyed, Animals
Abstract
Purpose: To test whether palisade endings are a general feature of mammalian extraocular muscles (EOMs).
Methods: Thirteen species, some frontal-eyed (human, monkey, cat, and ferret), and others lateral-eyed (pig, sheep, calf, horse, rabbit, rat, mouse, gerbil, and guinea pig) were analyzed. Palisade endings were labeled by using different combinations of immunofluorescence techniques. Three-dimensional reconstructions of immunolabeled palisade endings were done.
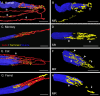
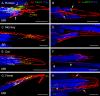
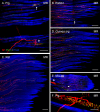
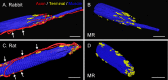
Results: In all frontal-eyed species, palisade endings were a consistent feature in the rectus EOMs. Their total number was high and they exhibited an EOM-specific distribution. In particular, the number of palisade endings in the medial recti was significantly higher than in the other rectus muscles. In the lateral-eyed animals, palisade endings were infrequent and, when present, their total number was rather low. They were only found in ungulates (sheep, calf, pig, and horse) and in rabbit. In rodents (rat, guinea pig, mouse, and gerbil) palisade endings were found infrequently (e.g., rat) or were completely absent. Palisade endings in frontal-eyed species and in some lateral-eyed species (pig, sheep, calf, and horse) had a uniform morphology. They generally lacked α-bungarotoxin staining, with a few exceptions in primates. Palisade endings in other lateral-eyed species (rabbit and rat) exhibited a simplified morphology and bound α-bungarotoxin.
Conclusions: Palisade endings are not a universal feature of mammalian EOMs. So, if they are proprioceptors, not all species require them. Because in frontal-eyed species, the medial rectus muscle has the highest number of palisade endings, they likely play a special role in convergence.
Figures




Comment in
-
Distribution of Palisade Endings Across Species Suggests They Function in Vergence Eye Movement Control.Invest Ophthalmol Vis Sci. 2016 Mar;57(3):948. doi: 10.1167/iovs.16-19151. Invest Ophthalmol Vis Sci. 2016. PMID: 26943157 No abstract available.
Similar articles
-
Molecular characteristics suggest an effector function of palisade endings in extraocular muscles.Invest Ophthalmol Vis Sci. 2005 Jan;46(1):155-65. doi: 10.1167/iovs.04-1087. Invest Ophthalmol Vis Sci. 2005. PMID: 15623769
-
Palisade Endings of Extraocular Muscles Develop Postnatally Following Different Time Courses.Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5105-5121. doi: 10.1167/iovs.17-22643. Invest Ophthalmol Vis Sci. 2017. PMID: 28986596
-
Axons giving rise to the palisade endings of feline extraocular muscles display motor features.J Neurosci. 2013 Feb 13;33(7):2784-93. doi: 10.1523/JNEUROSCI.4116-12.2013. J Neurosci. 2013. PMID: 23407938 Free PMC article.
-
Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity.J Anat. 2005 Mar;206(3):307-15. doi: 10.1111/j.1469-7580.2005.00378.x. J Anat. 2005. PMID: 15733303 Free PMC article. Review.
-
Palisade endings and proprioception in extraocular muscles: a comparison with skeletal muscles.Biol Cybern. 2012 Dec;106(11-12):643-55. doi: 10.1007/s00422-012-0519-1. Epub 2012 Oct 10. Biol Cybern. 2012. PMID: 23053430 Review.
Cited by
-
Extraocular Motoneurons and Neurotrophism.Adv Neurobiol. 2022;28:281-319. doi: 10.1007/978-3-031-07167-6_12. Adv Neurobiol. 2022. PMID: 36066830
-
A Subset of Palisade Endings Only in the Medial and Inferior Rectus Muscle in Monkey Contain Calretinin.Invest Ophthalmol Vis Sci. 2018 Jun 1;59(7):2944-2954. doi: 10.1167/iovs.18-24322. Invest Ophthalmol Vis Sci. 2018. PMID: 30025142 Free PMC article.
-
Does Surgical Resection of Horizontal Extraocular Muscles Disrupt Ocular Proprioceptors?Clin Ophthalmol. 2023 May 15;17:1395-1405. doi: 10.2147/OPTH.S381247. eCollection 2023. Clin Ophthalmol. 2023. PMID: 37214153 Free PMC article.
-
Mouse Extraocular Muscles and the Musculotopic Organization of Their Innervation.Anat Rec (Hoboken). 2019 Oct;302(10):1865-1885. doi: 10.1002/ar.24141. Epub 2019 May 7. Anat Rec (Hoboken). 2019. PMID: 30993879 Free PMC article.
-
Spinal cord from body donors is suitable for multicolor immunofluorescence.Histochem Cell Biol. 2023 Jan;159(1):23-45. doi: 10.1007/s00418-022-02154-5. Epub 2022 Oct 6. Histochem Cell Biol. 2023. PMID: 36201037 Free PMC article.
References
-
- Maier A,, DeSantis M,, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol. 1974; 143: 397–408. - PubMed
-
- Blumer R,, Lukas JR,, Aigner M,, Bittner R,, Baumgartner I,, Mayr R. Fine structural analysis of extraocular muscle spindles of a two-year-old human infant. Invest Ophthalmol Vis Sci. 1999; 40: 55–64. - PubMed
-
- Greene T,, Jampel R. Muscle spindles in the extraocular muscles of the macaque. J Comp Neurol. 1966; 126: 547–549. - PubMed
-
- Büttner-Ennever JA,, Konakci KZ,, Blumer R. Sensory control of extraocular muscles. Prog Brain Res. 2006; 151: 81–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

