Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma
- PMID: 26830441
- PMCID: PMC4740908
- DOI: 10.1038/ncomms10593
Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma
Abstract
Transdifferentiation (TD) is a recent advancement in somatic cell reprogramming. The direct conversion of TD eliminates the pluripotent intermediate state to create cells that are ideal for personalized cell therapy. Here we provide evidence that TD-derived induced neural stem cells (iNSCs) are an efficacious therapeutic strategy for brain cancer. We find that iNSCs genetically engineered with optical reporters and tumouricidal gene products retain the capacity to differentiate and induced apoptosis in co-cultured human glioblastoma cells. Time-lapse imaging shows that iNSCs are tumouritropic, homing rapidly to co-cultured glioblastoma cells and migrating extensively to distant tumour foci in the murine brain. Multimodality imaging reveals that iNSC delivery of the anticancer molecule TRAIL decreases the growth of established solid and diffuse patient-derived orthotopic glioblastoma xenografts 230- and 20-fold, respectively, while significantly prolonging the median mouse survival. These findings establish a strategy for creating autologous cell-based therapies to treat patients with aggressive forms of brain cancer.
Figures



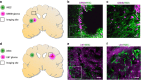




Comment in
-
Transdifferentiation Induced Neural Stem Cells for the Treatment of Malignant Gliomas.Neurosurgery. 2016 Aug;79(2):N17-8. doi: 10.1227/01.neu.0000489888.02840.33. Neurosurgery. 2016. PMID: 27428639 No abstract available.
Similar articles
-
Tumor-homing cytotoxic human induced neural stem cells for cancer therapy.Sci Transl Med. 2017 Feb 1;9(375):eaah6510. doi: 10.1126/scitranslmed.aah6510. Sci Transl Med. 2017. PMID: 28148846 Free PMC article.
-
Generation and Profiling of Tumor-Homing Induced Neural Stem Cells from the Skin of Cancer Patients.Mol Ther. 2020 Jul 8;28(7):1614-1627. doi: 10.1016/j.ymthe.2020.04.022. Epub 2020 Apr 29. Mol Ther. 2020. PMID: 32402245 Free PMC article.
-
Auto-loaded TRAIL-exosomes derived from induced neural stem cells for brain cancer therapy.J Control Release. 2024 Aug;372:433-445. doi: 10.1016/j.jconrel.2024.06.048. Epub 2024 Jun 26. J Control Release. 2024. PMID: 38908756 Free PMC article.
-
Reprogramming of somatic cells to induced neural stem cells.Methods. 2018 Jan 15;133:21-28. doi: 10.1016/j.ymeth.2017.09.007. Epub 2017 Sep 20. Methods. 2018. PMID: 28939501 Review.
-
Direct lineage conversion of astrocytes to induced neural stem cells or neurons.Neurosci Bull. 2015 Jun;31(3):357-67. doi: 10.1007/s12264-014-1517-1. Epub 2015 Apr 8. Neurosci Bull. 2015. PMID: 25854678 Free PMC article. Review.
Cited by
-
Early Changes in Tumor Perfusion from T1-Weighted Dynamic Contrast-Enhanced MRI following Neural Stem Cell-Mediated Therapy of Recurrent High-Grade Glioma Correlate with Overall Survival.Stem Cells Int. 2018 Mar 14;2018:5312426. doi: 10.1155/2018/5312426. eCollection 2018. Stem Cells Int. 2018. PMID: 29731779 Free PMC article.
-
Emerging Nano-Carrier Strategies for Brain Tumor Drug Delivery and Considerations for Clinical Translation.Pharmaceutics. 2021 Aug 3;13(8):1193. doi: 10.3390/pharmaceutics13081193. Pharmaceutics. 2021. PMID: 34452156 Free PMC article. Review.
-
Cell-based therapies for glioblastoma: Promising tools against tumor heterogeneity.Neuro Oncol. 2023 Sep 5;25(9):1551-1562. doi: 10.1093/neuonc/noad092. Neuro Oncol. 2023. PMID: 37179459 Free PMC article. Review.
-
Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signaling pathways.Oncotarget. 2017 May 27;8(43):74068-74095. doi: 10.18632/oncotarget.18240. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088769 Free PMC article.
-
Injectable Tumoricidal Neural Stem Cell-Laden Hydrogel for Treatment of Glioblastoma Multiforme-An In Vivo Safety, Persistence, and Efficacy Study.Pharmaceutics. 2024 Dec 24;17(1):3. doi: 10.3390/pharmaceutics17010003. Pharmaceutics. 2024. PMID: 39861656 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

