A phase 1 randomized, double-blind, placebo-controlled, crossover trial of DAS181 (Fludase®) in adult subjects with well-controlled asthma
- PMID: 26830468
- PMCID: PMC4736611
- DOI: 10.1186/s12879-016-1358-9
A phase 1 randomized, double-blind, placebo-controlled, crossover trial of DAS181 (Fludase®) in adult subjects with well-controlled asthma
Abstract
Background: Influenza virus (IFV) infection is associated with increased morbidity and mortality in people with underlying lung disease. Treatment options for IFV are currently limited and antiviral resistance is a growing concern. DAS181, an inhaled antiviral with a unique mechanism of action, has shown promise in early clinical trials involving generally healthy human subjects. This study was undertaken to assess the safety and tolerability of DAS181 in individuals with underlying reactive airway disease.
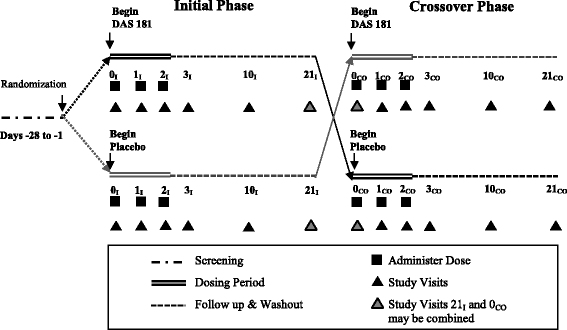
Methods: This was a randomized, double-blind, placebo-controlled, crossover phase 1 study of DAS181-F02. Dry particle inhaler administration of 10 mg was done on 3 consecutive days in ten adult volunteers with well-controlled asthma. The primary outcome was the frequency of adverse events (AEs), grade 1 or higher that occurred during each study period.
Results: There were 280 AEs among ten evaluable subjects (56.8 % active; 43.2 % placebo); 90.7 % were grade 1. No grade 3 or higher AEs occurred. A statistically significant association between exposure to DAS181 and experiencing any AE, a grade 1 AE, or a grade 2 AE was not detected. Overall, the majority of AEs were classified as possibly related (35.7 %), unlikely related (38.9 %), or unrelated (15.4 %) to study drug administration. However, there was a statistically significant association between exposure to DAS181 and experiencing a definitely or probably related AE. Respiratory effects, including dyspnea, dry cough, and chest discomfort related to respirations, accounted for all of the definitely related AEs and one of the most common probably related AEs.
Conclusions: DAS181 was safe in this small study of otherwise healthy subjects with well-controlled asthma. However, the generalizability of these results is limited by the small sample size and generally mild nature of the subjects' asthma at baseline. The increased association of respiratory events classified as probably or definitely related to DAS181 administration suggests caution may need to be employed when administering DAS181 to individuals with less stable reactive airway disease. Further investigation in a controlled setting of the safety and efficacy of DAS181 in a larger population of asthmatic subjects with varying disease activity is warranted.
Trial registration: ClinicalTrials.gov NCT01113034 Trial Registration Date: April 27, 2010.
Figures




Similar articles
-
Phase 1 clinical trials of DAS181, an inhaled sialidase, in healthy adults.Antiviral Res. 2015 Nov;123:114-9. doi: 10.1016/j.antiviral.2015.09.008. Epub 2015 Sep 25. Antiviral Res. 2015. PMID: 26391974 Free PMC article. Clinical Trial.
-
Efficacy and safety of inhaled zanamivir in the prevention of influenza in community-dwelling, high-risk adult and adolescent subjects: a 28-day, multicenter, randomized, double-blind, placebo-controlled trial.Clin Ther. 2007 Aug;29(8):1579-90; discussion 1577-8. doi: 10.1016/j.clinthera.2007.08.023. Clin Ther. 2007. PMID: 17919541 Clinical Trial.
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
A phase IIa proof-of-concept, placebo-controlled, randomized, double-blind, crossover, single-dose clinical trial of a new class of bronchodilator for acute asthma.Trials. 2018 Jun 18;19(1):321. doi: 10.1186/s13063-018-2720-6. Trials. 2018. PMID: 29914544 Free PMC article. Clinical Trial.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
Cited by
-
Pneumocystis colonization in asthmatic patients not receiving oral corticosteroid therapy.J Investig Med. 2017 Apr;65(4):800-802. doi: 10.1136/jim-2016-000381. Epub 2017 Feb 13. J Investig Med. 2017. PMID: 28193704 Free PMC article. Clinical Trial.
-
Effective in Vivo Targeting of Influenza Virus through a Cell-Penetrating/Fusion Inhibitor Tandem Peptide Anchored to the Plasma Membrane.Bioconjug Chem. 2018 Oct 17;29(10):3362-3376. doi: 10.1021/acs.bioconjchem.8b00527. Epub 2018 Sep 14. Bioconjug Chem. 2018. PMID: 30169965 Free PMC article.
-
Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season.Microbes Infect. 2020 Jul-Aug;22(6-7):236-244. doi: 10.1016/j.micinf.2020.05.005. Epub 2020 May 13. Microbes Infect. 2020. PMID: 32405236 Free PMC article. Review.
-
A Comprehensive Investigation Regarding the Differentiation of the Procurable COVID-19 Vaccines.AAPS PharmSciTech. 2022 Mar 21;23(4):95. doi: 10.1208/s12249-022-02247-3. AAPS PharmSciTech. 2022. PMID: 35314902 Free PMC article. Review.
-
Destabilization of the human RED-SMU1 splicing complex as a basis for host-directed antiinfluenza strategy.Proc Natl Acad Sci U S A. 2019 May 28;116(22):10968-10977. doi: 10.1073/pnas.1901214116. Epub 2019 May 10. Proc Natl Acad Sci U S A. 2019. PMID: 31076555 Free PMC article.
References
-
- Center for Disease Control. Updated CDC estimates of 2009 H1N1 Influenza Cases, Hospitalization and Deaths in the United States, April 2009-April 10, 2010. http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm. Accessed 24 June 2010.
-
- World Health Organization. Weekly update on oseltamivir resistance to pandemic influenza A(H1N1) 2009 viruses. 15 December 2010. http://www.who.int/influenza/2010_12_15_weekly_web_update_oseltamivir_re.... Accessed 29 December 2010.
-
- US Food and Drug Administration. Zanamivir Label Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021036s027lbl.pdf. Accessed 14 May 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

